MOJ
eISSN: 2381-182X


Mini Review Volume 4 Issue 2
Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hong Kong
Correspondence: Jian-Yong Wu, Department of Applied Biology and Chemical Technology, State Key Laboratory of Chinese Medicine and Molecular Pharmacology in Shenzhen, The Hong Kong Polytechnic University, Kowloon, Hong Kong, Tel +852 3400 8671, Fax +852 2364 9932
Received: January 28, 2017 | Published: April 3, 2017
Citation: Wu JY. Ultrasound-assisted extraction of polysaccharides from edible and medicinal fungi: major factors and process kinetics. MOJ Food Process Technol. 2017;4(2):48-52. DOI: 10.15406/mojfpt.2017.04.00086
Polysaccharides (PS) from medicinal fungi (mushrooms) provide an important source of nutraceutical and pharmaceutical compounds with notable antitumor, immunomodulatory and other health benefits.Extraction (solid-liquid) is the first key step for isolating PS from the fungal materials. Power ultrasound (US) is a special and favorable means for processing food and medicinal products becuase of several merits such as simple equipment, lower temperature and less use of chemicals. The various effects of power US can be mostly attributed to the hydrodynamic activities of acoustic cavitation, leading to cell wall disruption and more effective mass transfer. The major factors affecting the rate of US-assisted extraction (UAE) include the solid particle size, solid-liquid ratio; US power intensity and temperature, plus the physical properties of solid materials being extracted. Besides the extraction process (rate and yield), US may also affect the properties and bioactivities of extract products. This article is to give a brief review of UAE for extraction of PS from edible and medicinal mushrooms.
Keywords: extraction, ultrasound, polysaccharide, mushroom, process factors, kinetic model
Mushrooms are favorite foods in our daily diet, many having tonic effects and medicinal properties. Polysaccharides (PS) are major functional constituents of edible and medicinal mushrooms (fungi), which are well known for their antitumor and immunomodulatory activities and other bioactivities such as anti-inflammation, hypoglycemic, hypolipidemic, antioxidant, and antiviral.1-3
Some mushroom PS have been used commercially as immunotherapeutic agents and adjuvant drugs for cancer therapy and PS-rich extracts of mushroom fruit bodies or fungal mycelia are widely used in functional foods.4 Unlike the small antitumor molecules with strong cytotoxicity, most of the mushroom PS have low toxicity and produce antitumor effects by acting as biological response modifiers or immunomodulators on the host immune responses.5
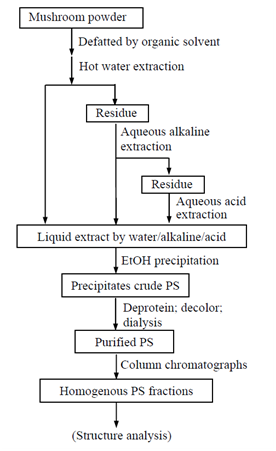
Extraction is the first key step for separation of PS from mushrooms as well as other material sources. The specific extraction procedure and conditions depend on the PS structure and water-solubility and (Figure 1) shows a general procedure for extraction, isolation and purification of polysaccharides from mushrooms. In brief, the dry mushroom or fungal mycelium in the form of dry powder is first defatted with an organic solvent such as acetone and ethanol to remove water-insoluble organic components. The defatted mushroom is extracted successively with boiling water, hot aqueous alkaline solution, and hot aqueous acid solution.6,7 The liquid extract from each extraction step is precipitated with ethanol to attain the crude PS. The crude PS is purified though a series of steps for removal of proteins, pigments and other low molecular weight components. The purified PS can be further purified by ion-exchange and gel permeation chromatography to retain homogenous PS fractions for structure analysis or other uses. The molecular structure of purified PS fractions can be determined through specialized protocols involving extensive degradation reactions and analytical measurements.
Power ultrasound (US) is reagreded as a special and favorable means for processing food and medicinal products becuase of several merits such as simple equipment, direct and quick effect (fast start-up), clean and safe operation (milder conditions, e.g. lower temperature and less use of chemicals).8-10 Ultrasound-assisted extraction (UAE) has been widely exploited to enhance or replace the hot-water and solvent extractions for the separation of bioactive components from food and medicinal products.11-13 The potential advantages of UAE over the conventional methods for separation of bioactive natural products include higher extract yield and extraction rate, and lower extraction temperature. The low-temperature operation of UAE is most favorable for preserving the properties and activities of heat-sensitive food and medicinal products which could be deteriorated in hot water or solvent extraction processes. UAE is also more favourable than other non-conventional techniques for laboratory analysis and large-scale separation of food and medicinal products in the simplicity, energy efficiency, scalability, versatility, safety and environmental compatibility of equipment and operation.9,10 While most early studies on UAE of food and natural products were aimed at the small organic molecules, many recent studies have applied power US to improve the extraction of natural PS from plants, mushrooms and other sources.
Power ultrasound (US) is a versatile tool in the laboratory and industry for various processes such as cleaning, extraction, homogenization and chemical reactions.14 Ultrasound-assisted extraction (UAE) is the application of high-intensity power US to enhance the extraction of solid constituents in a liquid solvent. Practically UAE has been applied in the laboratory for a long time for extraction and homogenization of chemical, food and biological samples with ultrasonic cleaning baths or probe horns, often as a routine sample preparation step prior to analysis and measurement.15 However, the application of UAE in large-scale commercial processes of food and natural products is still classified as a non-conventional process.8,16,17
Most previous studies on UAE of natural products have been aimed at the low-MW organic molecules such as phenolic and aromatic compounds, alkaloids and essential oils from plants and various other sources.12,18 With increasing interest in the bioactivities and other useful properties of natural PS, more recent studies have devoted to UAE of PS from plant materials such as herbs and agriculture residues19,20 and from edible/medicinal mushrooms such as Lentinusedodes (Shiitake),21,22 Ganodermalucidum (Lingzhi),23 Agaricusbisporus (button)24 and Cordycepssinensis (caterpillar fungus) mycelium.25 These studies suggest that UAE is a viable alternative to classical extraction methods such as hot water extraction (HWE) for isolation of PS from various sources. A specific concern for UAE of PS and related biopolymers is depolymerizationwith high intensity US and long exposure period.
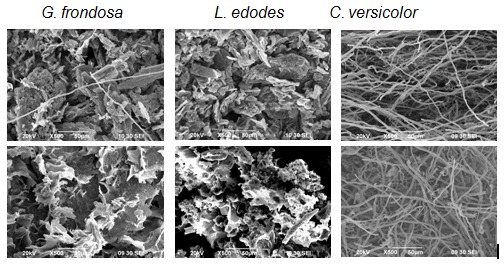
We have accomplished several studies on UAE of PS and PS-protein complexes (PSPs) from selected species of the most important and valuable edible and medicinal fungi in both fruiting body and mycelia form.26-28 In these studies, power US was applied to the powdered samples dispersed in water with an ultrasonic probe horn at a fixed power (Figure 2). During UAE, the sample bottle was immersed in ice to avoid overheating and maintain a low temperature (<50°C). In one study with three species of mushrooms, the yield and extraction rate of PSP by UAE was notably higher with G.frondosaand only slightly higher with L. edodes, but even lower with C. versicolor, than by HWE (Table 1). The different effects of UAE on the efficiency of PSP extraction from the three mushroom species was mainly attributed to the different physical properties of mushroom tissue and the aggregation or dispersion of mushroom powder in the extraction liquid. In particular, the powder G. frondosa and L. edodessamples subjected to UAE were in the form of small, well-dispersed particles in water but the C. versicolor powder formed large, irregular aggregates (Figure 3). Under scanning electron microscopy (SEM), the G. frondosa and L. edodesmushroom powder appeared as irregular particles before UAE and became looser and more porous after UAE, while the C. versicolor powder was in fibrous form before and after UAE (Figure 4).
Mushroom |
PS Yield |
Carbohydrate |
Protein |
|||
UAE |
HWE |
UAE |
HWE |
UAE |
HWE |
|
G. frondosa |
6.30± 0.07 |
5.71± 0.07 |
28±0.4 |
60±4.8 |
33±2.9 |
21±0.3 |
C. versicolor |
1.61± 0.07 |
6.58± 0.04 |
21±0.5 |
66±3.0 |
25±0.3 |
15±0.7 |
L. edodes |
12.4± 0.70 |
6.57± 0.05 |
36±1.4 |
46±1.9 |
34±0.3 |
29±0.1 |
Table 1 Yield and composition of PS extracted from three mushrooms (fruit body) by UAE and HWE (UAE at < 50°C; all values in wt%).UAE was performed with a probe horn ultrasonic processor of 20 kHz frequency and 130W power (Sonics & Materials Inc., Newton, USA)26

Our studies have also shown significant differences in the composition and molecular properties between the PSPs attained by UAE and those by HWE.26 Compared with those extracted by HWE, the PSPs from all the three mushrooms extracted by UAE had much higher protein contents and lower carbohydrate contents (Table 1). The molecular weight distribution spectra of PSPs from UAE showed an overall shift of the peaks to lower molecular weight range, which was due probably to the lower extraction temperature and/or partial degradation of the PSPs by the US power.26 In addition, only the PSPs extracted by UAE but not by HWE exhibited distinct protein bands on SDS-PAGE, suggesting that UAE at low temperature retained the protein constituents which could be denatured during HWE. The PSPs by UAE from three mushrooms also exhibited higher antioxidant activities.
Ultrasound-assisted solid-liquid extraction is a complex physical process depending on several classes of factors, namely ultrasonic (equipment structure and operation), solid material (physical properties, particle size and morphology), liquid solvent (property and volume ratio), and environmental(temperature, agitation).Ultrasonic enhancement of extraction is attributed mostly to disruption of cell walls, reduction of particle size and enhanced mass transfer through the solid particle by the hydrodynamic activities of cavitations’ bubbles.29 The kinetic models for UAE of natural products are usually adapted from those for conventional solid-liquid extraction or leaching, a well-known mass transfer process in chemical engineering. Several simplified empirical models have been developed for describing the extraction kinetics of natural products from plant materials in various mathematical forms, parabolic, hyperbolic, power-law and exponential.30 The experimental data (extract yields versus time) of UAE of mushrooms and fungal mycelia in our previous studies were fitted to several kinetic models for solid-liquid extraction.27,28 Based on the R2 values from linear regression (Table 2), three models including the power law, Weibull exponential model and Elovich logarithmic model, fitted closely to the data for fruit body of G. frondosa and L. edodes, and the parabolic diffusion model showed a close fit for all mycelia, Cs-4, Cs-HK1 and G. lucidum, and no model fitted well for C. versicolor fruit body.
|
Fruit body |
Mycelium |
||
Kinetic model |
G. frondosa |
L. edodes |
C. versicolor |
Cs-HK1 |
Parabolic diffusion: |
0.907 |
0.871 |
0.778 |
0.995 |
Power Law: |
0.984 |
0.964 |
0.559 |
0.926 |
Weibull: |
0.984 |
0.964 |
0.559 |
0.913 |
Elovich: |
0.984 |
0.963 |
0.564 |
0.913 |
Unsteady diffusion: |
0.743 |
0.717 |
0.823 |
0.906 |
Table 2 The linear regression coefficients (R2 values)for the UAE data (total extract yields versus time) of various mushroom materials fitting to different kinetic models27
Power US is a convenient and effective means for improving the extraction of bioactive natural products in both laboratory and industry applications. For extraction of PS from mushrooms, high-intensity US affects not only the extraction rate and yield but also the molecular composition and properties of PS. The US effect on the extraction process is dependent on the microstructure of mushroom particles and their dispersion in the exacting solvent, in addition to the process factors such as the solid particle size, solid-liquid ratio, US power intensity and temperature. The UAE process kinetics of mushrooms can be well represented by simple empirical kinetic models for solid-liquid extraction. These models are useful for prediction and optimization of the UAE process factors and also for understanding the mechanisms of ultrasonic effects on the solid-liquid mass transfer process.
None.
The author declares no conflict of interest.

©2017 Wu. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.