MOJ
eISSN: 2381-182X


Research Article Volume 4 Issue 6
Department of food science and technology, Kwame Nkrumah university of Science and Technology, Ghana
Correspondence: Jacob K Agbenorhevi, Department of food science and technology, Kwame Nkrumah university of Science and Technology, Kumasi, Ghana, Tel +233 208954223
Received: July 20, 2016 | Published: August 15, 2017
Citation: Osae AK, Agbenorhevi JK, Manu FDW. Packaging and shelf life of maize-peanut balls (Dakua). MOJ Food Process Technol. 2017;4(6):162-166. DOI: 10.15406/mojfpt.2017.04.00108
Maize-peanut balls (also known as dakua, dzowe or donkwa) is a street snack made from roasted, dried and milled maize and groundnuts/peanuts. It is often sold unpackaged by hawkers/vendors, in a portable food booth, food cart or food truck. However, the shelf life is unknown which makes this nutritious snack also a potential risk to consumers’ health. In this study, the packaging and shelf life of maize-peanut balls (lab/self-made and those purchased from the local markets) were investigated by packaging in aluminum foil, flexible plastic films and unpackaged and stored at ambient temperature for 5weeks. The products were analysed weekly for moisture, peroxide value, aflatoxin level, yeast and moulds during storage. Results indicate that the samples had variable moisture content, which decreased from the start of the analysis to the third week and increased again. Over the 5weeks storage period, peroxide values increased to ~0.4mEq/kg. The unpackaged samples had the highest aflatoxin level (~21.74ppb), which is above the acceptable limit of 20ppb. The samples packaged in flexible plastic film, however, had the lowest aflatoxin level (~13.15ppb) whereas samples packaged in aluminum foil had values of ~16.75ppb. The estimated shelf life is approximately 8weeks with flexible plastic films as the most suitable packaging material.
Keywords: dakua, dzowe, donkwa, groundnut-maize balls, shelf life, processing, packaging, HPLC, WHO
Maize-groundnut balls (also known as dakua in Akan and dzowe in Ewe) originated from Nigeria where it is known as donkwa and has been adopted by the Voltarians in Ghana. Dakua is a traditional food1 basically made from groundnuts2,3 maize and some spices (Figure 1). It is a street food, simply because it is a ready-to-eat food sold in the street or any public place such as the market by a hawker or vendor, normally in a portable food booth, food cart or food truck.4
Dakua is nutritious due to its maize and groundnut content. These constituents enrich the product and cause it to be highly nutritious.5 In Ghana, like other snack foods, dakua is mainly produced and consumed in its areas of production and production is based on art and skills rather than scientific knowledge. Production varies with people, culture and geographical locations: these leads to possession of variable characteristics.6 Some of the problems associated with the local production of snacks such as dakua include non-standardization of equipment, process and raw material, inadequate hygiene during and after production, and little or no packaging which results in poor preservation techniques and high levels of contaminants in the food resulting in food borne illnesses.7
Dakua is a street snack consumed by many due to its nutritional value. However, a lot more people are concerned about the way dakua is handled after processing before it is sold to consumers. This also leads to high levels of contaminants in the food resulting in food borne illnesses. Due to this, many people do not patronize this snack in bulk because of the little knowledge of its shelf-life with respect to packaging. A well-developed storage plan for Dakua is one that will therefore be useful to both producers and consumers; where the glossy appearance, smooth texture, suitable peroxide value, aflatoxins and nutritional value is maintained over a longer period of time. The main objective of this study is to develop a suitable packaging for the dakua snack and also to evaluate the effects of these packages on shelf-life.
Materials and sample preparation
Samples were obtained from 3 sources: Kwadaso area, Tech junction and those prepared in the food science kitchen, Forig, KNUST. Samples were stored at room temperature. Dakua (Sample C) was produced (according to the Flow chart as shown in Figure 1) using roasted Maize (900g), roasted Groundnut (900g), sugar (600g), ginger (250g), dried red pepper (50g), Ethiopian pepper (50g), nutmeg (50g), gloves (40g) and salt (10g).
Products were also obtained from 2 different sources (A = Kwadaso ; B = Tek Junction).
Maize grains and groundnut were manually cleaned. They were then washed in tap water and 400 g each were soaked in 2 litres of water for 12 hours after which they were germinated for 72 hours. After germination, the maize and groundnut seeds were oven dried at 105˚C for sixty minutes. The groundnut seeds were roasted at 140˚C for thirty minutes while the maize was roasted at 140˚C for sixty minutes. The groundnut was de-coated, after which both maize and groundnut were milled separately using a local attrition mill. After milling, the maize flour was sieved to obtain a particle size of 0.05 mm. The maize flour and groundnut paste were then mixed together in a 1:1 ratio. To every 100 g of this mixture, 10 % and 5 % respectively of table sugar and powdered red pepper were added. The mixture was then passed through the attrition mill once and moulded into balls. The Dakua balls were then packaged in aluminum foil and flexible film while some were left unpackaged. The packaged and unpackaged samples were stored in a ‘sieve’ at ambient temperature.8 The products were analyzed for moisture, peroxide value, aflatoxin, yeast and moulds every week using standard procedures.9-11 The shelf-life was estimated using Statsgraphics Centurion software (2008).
Isolation and enumeration of yeasts and moulds
Ten grams of the sample was weighed and homogenized with 90 mL of Saline Peptone Solution in a stomacher. Suitable serial dilutions were made. One ml was inoculated in Petri dishes. 15 mL of molten medium (cooled to 45±1°C) was poured into the Petri dishes, mixed and allowed to set, incubated at 25°C for 3 days and then counted under a microscope with oil immersion objective lens.9
Aflatoxin determination
The aflatoxin content was determined by means of high performance liquid chromatography (HPLC). Fifty grams of ground sample were placed into ultraturrax and then 100 mL of 60 % acetonitrile/water (v/v) added. The mixture was stirred for 2 min at high speed. The extract was filtered through a Whatman No. 3 filter paper and then through a microfiber filter. Two milliliters (2 mL) of the final extract, corresponding to 1 g of the original material was diluted with 48 mL of phosphate buffered saline (PBS, pH 7.4, R-Biopharm Rhone) to give a solvent concentration of 2.5 % or less (in order to protect the antibodies in immunoaffinity columns). The mixture was allowed to pass through column at a flow rate of 5 mL/min. The column contains monoclonal antibodies to aflatoxins bound to a solid support. By passing the diluted extract through column any aflatoxins present in the sample are bound to the antibody within the column. The column was then washed with 20 ml of PBS. The elution of aflatoxins was done with 1.5 mL of methanol and 1.5 mL of pure water. 100 μL of the samples were injected into the HPLC column heated to 40°C. The mobile phase was water: methanol solution (60:40, v/v). To 1 L of mobile phase were added 119 mg of potassium bromide and 350 μL of 4 M nitric acid. The flow rate was 1 mL/min. For fluorescent detection of aflatoxin B1 & B2, the excitation wavelength was 362 nm whereas the emission wavelength was 425 nm.11
Peroxide value
The soxhlet method was used for the extraction of the peanut oil from the Dakua samples. The peroxide value determination was performed on the extracted oil by means of AOAC Official method.12
Enumeration of yeasts and moulds
The quantitative analysis of yeasts and moulds were determined every week for 4 weeks. According to the World Health Organisation (WHO), the accepted level of yeasts and moulds in nuts is 5×103 CFU/g. An increase in yealts and mold growth was observed in all the samples of both packaged and unpackaged (Figure 2). For source A, the fresh samples recorded values of less than 10 CFU/g. The unpackaged sample increased to 120 CFU/g during the 2nd week, there was a sharp increase to 180 CFU/g and gradually to 210 CFU/g during the 4th week. For the samples packaged in plastic, the increase was from an insignificant value of less than 10 CFU/g to 110 CFU/g and then 150 CFU/g in the 3rd week and finally 190 CFU/g in the 4th week. The samples packaged in aluminum foil recorded the lowest levels after every week. The samples had an initial microbial load of less than 10 CFU/g which is insignificant and this increased to 100 CFU/g and then to 130 CFU/g and finally to 160 CFU/g after the 4th week. For source B, there was also an initial microbial load of less than 10 CFU/g which increased over a period of 4 weeks. For the unpackaged samples, the load increased from 0 to 190 CFU/g, 250 CFU/g and lastly 290 CFU/g after the 4th week. The samples packaged in aluminum also recorded values from 0 to 120 CFU/g, 160 CFU/g and after the 4th week, 210 CFU/g. The samples packaged in plastic also had the same trend, that is, from 0 to 140 CFU/g, 170 CFU/g and 220 CFU/g after the 4th week. For sample C, initially, the yeasts and moulds load was insignificant and this increased after every analysis. After the 2nd week, the unpackaged samples recorded a value of 200 CFU/g then increased to 210 CFU/g and finally to 275 CFU/g after 4 weeks. The sample packaged in aluminum foil also increased to 160 CFU/g, 175 CFU/g and finally, 240 CFU/g after the 4th week. Finally, the samples packaged in plastic recorded 170 CFU/g after the 2nd week, 192 CFU/g after the 3rd week and after the 4th week, 255 CFU/g.
From the results, it was noticed that the samples which were not packaged had a higher microbial load as compared to the others. This is due to the exposure of the samples to the atmosphere thereby adding on to the aerobic microbial load which multiply faster as compared to the anaerobic microbes. The increasing microbial load in the packaged samples may be due to the presence of anaerobic microbes. These microbes are otherwise known as facultative microbes and do not require energy for their growth and activities. There was microbial growth in all the samples with very similar trend and this increased as the weeks went by. Both samples seemed to have favourable conditions for the growth of the fungi present, be it anaerobic or aerobic. The microbes produce toxins and these toxins accumulate may cause cancerous effect when consumed over a period of time.
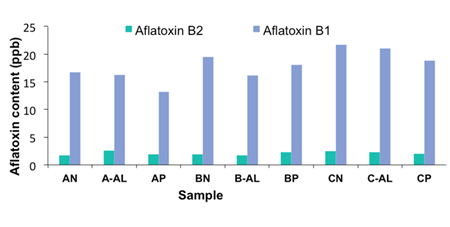
Figure 2 AN: source A no packaging; A-AL: source A aluminium packaging; AP: source A plastic packaging; BN: source B, no packaging; B-AL: source B, aluminium packaging; BP: source B, plastic packaging; CN: source C, no packaging; C-AL: source C, aluminium packaging; CP: source C, plastic packaging.
Aflatoxin levels
The aflatoxin level was determined after the 3rd week where an increase in the number of colonies of yeasts and moulds was vividly seen. Aflatoxins are produced by Aspergillus flavus and Aspergillus parasiticus, species of fungi. They are said to be carcinogenic substances which occur naturally in the peanut.
The aflatoxins B1 and B2 were detected. Aflatoxin 13,14 B1 is the most toxic and frequently detected form. The level of aflatoxin increases as the colonies of these causative fungi also increase. From the results, it was noticed that the aflatoxin level at the 3rd week was seen to be above the accepted WHO level which is 15 ppb. The levels were either slightly above or nearing the accepted Food and Drugs Authority, Ghana level, which is 20 ppb (Figure 3). The highest level was recorded in the unpacked sample from source C which recorded a value of 21.74 ppb followed by the source C sample packed in aluminum foil which read a value of 21.05 ppb. These values maybe be due to the handling of the raw peanut, from the farmer to the Dakua producer. The peanut, when handled improperly would encourage the growth of the aflatoxin producing fungi. It could also be due to the degree of processing done. This could be the peanut which may not have been processed enough to eliminate these toxins or better still kill the fungi which produce these toxins. The least levels recorded were from source A. The samples packed in plastic recorded the lowest level of 13.15 ppb. The samples packaged in aluminum foil recorded values of 16.75 ppb and 16.28 ppb, respectively. This could also be related to the better handling of the raw material as compared to source C and also the degree of processing done to the raw materials.
Moisture content
Moisture content was recorded every week for samples from all 3 sources. The amount of moisture in the sample is important as it is a growth factor for microbes that might be present in a food sample. The amount of moisture would either support or hinder the growth of these microbes. Samples from Kwadaso had the highest initial moisture content recorded it had a value of 10.01%. Samples from the food science kitchen recorded a value of 8% and samples from tech junction recorded a value of 7.03%. The moisture content in all the samples followed the same trend. For the unpackaged samples, the moisture content decreased at a steady rate throughout the research. This was due to the loss of water into the atmosphere, in a form of water vapour, as a result of temperature change. The sample loses moisture to the atmosphere when the temperature of the sample is higher than that of the atmosphere. For the packaged samples, the moisture level was seen to have decrease to a point and after 3 weeks, it started to increase again. This was due to the fact that the samples were enclosed in a package, therefore when the sample loses moisture the moisture is trapped in the package. When the temperature reduces, the water vapour condenses and falls back on the sample, therefore adding on to the moisture content of the sample. The increase could also be attributed to the presence of anaerobic microbes. These are microbes which have the tendency to survive and indulge in their normal activities and functioning without the use of oxygen. These are the only microbes which would survive in a sample enclose in a tightly sealed package. These microbes produce alcohol and water and this water adds on to the moisture content of the sample. Basically, in the packaged samples, more moisture is present in the sample as these microbes increase and the temperature fluctuates whereas in the unpackaged samples, moisture is constantly lost. These microbes also produce alcohol and water as their by-products, the water also adds on to the quantity on water already available in the samples, thereby attributing to the increase of moisture after it has reduced or been used up by these microbes.7
Peroxide value
The peroxide value which is usually used as an indication of deterioration of oil measures the primary oxidative products (peroxides) of fat and oil.15 The peroxide values of the oil samples were relatively low. Over the 5 weeks storage, peroxide values increased to ~0.4 meq/kg (Figure 4). The low peroxide values of the samples are very stable and do not undergo rancidity at a fast rate and thus give an indication that peanut oil has a good storage potential.16,17
Shelf life
The shelf life was calculated using the Statsgraphics Centurion software (Stat Point, 2008). The results from the yeasts and moulds was used for the estimation shelf life.
The samples which were not packaged had lower shelf life compared to their corresponding packaged samples, except for source C (Table 1). This was due to the fact that the packaged source C samples recorded a higher microbial18 load as compared to the unpackaged ones. This could be attributed to the fact that the samples contained more facultative or anaerobic microbes and compared to the aerobic ones. It can also be traced to the raw materials or the environment in which the samples were produced. It was also noticed that the samples packaged in plastic had a lower shelf life as compared to those packaged in aluminum foil.19 Plastic is known to have micropores in the material so the product in the plastic can support the anaerobic microbes when there is not enough oxygen for their growth and activities and also the aerobic microbes when the material allows just enough oxygen into the packaged sample.
Samples |
Shelf- life (weeks) |
A-NP |
9.83 |
A-AL |
11.39 |
A-PL |
10.62 |
B-NP |
5.89 |
B-AL |
8.57 |
B-PL |
10.02 |
C-NP |
8.87 |
C-AL |
5.5 |
C-PL |
5.78 |
Table 1 Shelf life estimated for each sample
AN: source A no packaging
A-AL: source A aluminium packaging
AP: source A plastic packaging
BN: source B, no packaging
B-AL: source B, aluminium packaging
BP: source B, plastic packaging
CN: source C, no packaging
C-AL: source C, aluminium packaging
CP: source C, plastic packaging
From this study, it was noticed that the initial or raw materials used for the production of the product greatly affects the shelf life. Also, the conditions around the processing area also affects the shelf life. For instance, samples from source C were exposed to more anaerobic microbes and this affected the analysis done thereby causing the different direction in which the shelf life took. Therefore, Dakua can last for between 5 to 10 weeks depending on the packaging20,21 used.
The shelf life of Dakua was significantly influenced the nature of packaging. Dakua samples had variable moisture content during storage in the different packaging studied. The unpacked samples had the highest aflatoxin level (~21.74 ppb), which was above the acceptable limit of 20 ppb (Food and Drugs Authority, Ghana). The samples packaged in flexible plastic film, however, had the lowest aflatoxin level (~13.15 ppb) whereas samples packaged in aluminum foil had values of ~16.75 ppb. The estimated shelf life is approximately 8 weeks with flexible plastic films as the most suitable packaging material.
The authors are grateful to William Appaw of the Mycotoxin and Food Analysis Laboratories- KNUST for the technical support with the aflatoxin analysis.
The author declares no conflicts of interest.

©2017 Osae, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.