MOJ
eISSN: 2381-182X


Research Article Volume 2 Issue 4
Laboratory of Physiopathology and Molecular Genetic, Hassan II University of Casablanca, Morocco
Correspondence: EL Khasmi Mohammed, Laboratory of Physiopathology and Molecular Genetic, Faculty of Sciences Ben MSik, Hassan II University of Casablanca, P.B 7955 Sidi Othmane, Casablanca, Morocco, Tel 213000000000, Fax 213000000000
Received: May 21, 2016 | Published: June 2, 2016
Citation: Kaoutar B, Mohamed F, Fouad R, et al. Impact of transport distance on some stress biomarkers levels in camel meat. MOJ Food Process Technol. 2016;2(4):130-134. DOI: 10.15406/mojfpt.2016.02.00043
Animals intended for slaughter are subjected to several stress factors which can induce harmful effects on physiological functions as well as the quality of their meat. Road transport is one of the major stressors that affect the well being of the animal. The intensity of the stress depends on several factors, namely the density of the load, the ambient temperature, relative humidity, topography and the distance of transportation. The aim of this investigation was to study the effect of transport distance on some physicochemical parameters and stress biomarkers in the meat of camel (Camelus dromedarius). Fourteen camels were selected and divided into 2 groups of 7 individuals, depending on distance traveled during transport from Settat (short distance of 72Km) or Beni-Mellal (long distance of 160km). The water content, dry matter, ash and proteins in 3 muscles (triceps, oblique and diaphragm) showed no significant variation with distance transportation. While the rate of glycogen decreases and pHu increased significantly in these muscles, when the distance of travel increases. The level of malondialdehyde increases while the catalase activity decreases significantly in all three muscles when the transport distance increases. Our findings could be explained at least partly by the effect of preslaughter stress induced by transport resulting in stress oxidant activity in camel meat rising much with road distance.
Keywords: catalase, dromedary, dry matter, glycogen, malondialdehyde, meat, phu, proteins, road transport, stress
CAT: Catalase; MDA: Malondialdehyde; ORS: Oxygen Reactive Species; OS: Oxidant Stress; pHu: Ultimate pH
Domestic animals destined for meat production are usually exposed to preslaughter stress-full conditions such as handling, loading and unloading, transportation time and distance, stocking densities, passing through livestock markets, fasting and lairage that affect their welfare and meat quality.1 During environmental stress, marked changes in the levels of reactive oxygen species (ROS) scavengers occurred in the serum of camels.2 As a very stressful factor, road transport of camels to the slaughterhouse is able to induce a significant increase of circulating levels of cortisol and oxidant stress (OS) indicators3,4 resulting in an increase of free radical generation5 and showing a significant correlation with road transport distance.6 Furthermore, the reaction to transport stress may have an impact on the quality of slaughtered animal meat and depends on the duration and intensity of this stressor.7,8 The objective of the study was to investigate the effect of transport distance on the ultimate pH (pHu: 24h postmortem pH), and the levels of water, ash, glycogen, protein and OS indicators (MDA and CAT)in meat of dromedary camels.
Animals
The effect of travel distance on some OS indicaotrs in meat was assessed in fourteen 4-5-year old male Moroccan dromedary camels (Camelus dromedarius) weighing 290-320 kg. The animals were divided in two groups I and II of seven camels according to the preslaughter road transport distance (72 km or 160 Km respectively) to Casablanca Municipality slaughterhouse in Morocco. All animals were clinically healthy and feed deprived overnight and was transported at average temperature of around 25-30 °C and relative humidity of around 55-65%.
Tissues collection
After veterinary inspection, samples of muscles (150-200g) (Triceps brachii, Musculus obliquus and Diaphragma) were collected to the left side in municipal slaughter house of Casablanca at about 10 am using a sharp knife at a depth of 2 to 3cm. The tissue samples were taken aseptically at 4°C in a cooler to the laboratory of Molecular Genetic and physiopathology in faculty of Sciences Ben M’Sik in Casablanca. The tissues were divided into 3 parts: one to measure the pHu, the other to analyze water and ash, and the last to determine the levels of glycogen, protein and OS indicators (MDA and CAT).
pH Measure
The extent of muscle pH was performed directly using a calibrated pH meter on the extracted meat samples crushed and homogenized using a porcelain mortar.
Humidity and Ash
Moisture tissue was determined by desiccation of a test sample in an oven at 105 °C for 24h until a constant weight. The rates of solids (S%) and moisture (Water %) were determined by the difference of weight as follows:
S% = (sample mass/dry mass of fresh sample) x100,
and Water % = 100 - S%.
The rate of total ash was obtained by incineration. After baking at 105 °C for 24h the meat samples were incinerated in a muffle furnace (1h at 600 °C). The ashes were evaluated by the difference in weight.
Total ash% = (ash mass/mass of dry sample)x100.
Preparation of the homogenate
Each tissue were placed in the presence of a phosphate buffer solution (0.1M, pH 7.4) (500mg/5ml), then were ground in cold (4°C) using an ultra shredder for 30 seconds to obtain a homogenate, it is centrifuged at 5000 rpm for 15 minutes. Homegenates were stored at -80°C until analysis of MDA and CAT activity.
Glycogen analysis
Glycogen assay was performed by colorimetric method.9 The iodine contained in the reactive iodine water is adsorbed on the polysaccharides formed glucopyranose associated by α1-4 linkages, at 2 iodine molecules per spiral turn. Ten grams of muscle were cut into small pieces, ground and then boiled in 50 ml of distilled water for 2 min. The tissue fragments were drained using a sieve and milled with mortar. 25 ml of distilled water were added to the homogenate and the suspension was boiled for 5 min. The bouillonnât was filtered and the filtrate was recovered with 3 drops of HCl and was filtered again. The filtrate was treated with 4 times its volume of alcohol 95%, filtered and the final filtrate was taken up with 2 ml of distilled water then stored at -80°C until analysis. For the determination of glycogen, a drop of iodine water was added to 1 ml of extract of glycogen and the optical density of the mahogany-brown color was read at 470nm. Glycogen concentration was deduced from a standard range established with the pure glycogen as standard.
Protein dosage
The assay was performed by the biuret method.10 Proteins form a colored complex in the presence of copper salts in alkaline medium according to the reaction of biuret. The intensity of the resulting color is proportional to the protein concentration.
Malondialdehyde dosage
The assay was performed by the method of Ohkawa et al.11 The assay based on the formation of acid and heat medium (100°C) between MDA and thiobarbituric acid (TBA) an absorbent colored pigment at 530 nm, extractable by organic solvents such as butanol. 0.5 mL of the homogenate was mixed with 0.5 ml of trichloroacetic acid (TCA) 20% and 1 ml of thiobarbituric acid (TBA) 0.67%, then incubated in a water bath at a temperature of 100°C for 15 minutes. After cooling, 4 ml of n-butanol was added to the mixture and then centrifuged for 15 minutes at 3000 rpm. And finally the optical density of the supernatant was measured at a wavelength equal to 530nm against the blank.
Catalase activity analysis
The CAT activity was measured using the method of Aebi.12 The disappearance of hydrogen peroxide was monitored spectrophotometrically at 240nm for 5min. A molar extinction coefficient of 0.041/mM/cm was used to determine the CAT activity. The activity was defined as the µmol decreased H2O2/min/mg protein.
Statistical analysis
The data were expressed in SI. All values were expressed as Mean ± standard error (ET) and analyzed by the Mann-Whitney U test for comparison between groups. The degree to which variables were related is measured with Pearson’s correlation. P values greater than 0.05 were considered insignificant.
In the work reported here, transport distances showed no significant differences of water, solids, ashes and protein contents in all studied muscles (Triceps brachii, Musculus obliquus and Diaphragma) (Table 1).These muscles showed a significant (P<0.05) increase of pHu at 160Km compared with 72Km (respectively 6.4±0.2 vs 5.7±0.2; 6.5±0.2 vs 5.6±0.1 and 6.3±0.1vs 5.6±0.2) (Figure 1) and a significant increase of MDA levels (µMoles/Kg) at high distance compared with short one (respectively 0.181±0.014 vs 0.124±0.011; 0.175±0.014 vs 0.133±0.013 and 0.179±0.011 vs 0.131±0.012) Table 2). However, in the muscles (Triceps brachii, Musculus obliquus and Diaphragma) the glycogen levels (mg/100g) decreased significantly (P<0.05) at high distance compared with short one (respectively 170±20 vs 226±25; 191±21 vs± 241±27 and 180±23 vs 237±25) (Figure 2), and a significant (P<0.05) decrease of the CAT activity (UI/g) at the same conditions and with the same way in these muscles was observed (respectively 337±37 vs 440±44; 326±31 vs 410±41 and 330±33 vs 432±50) (Table 2).
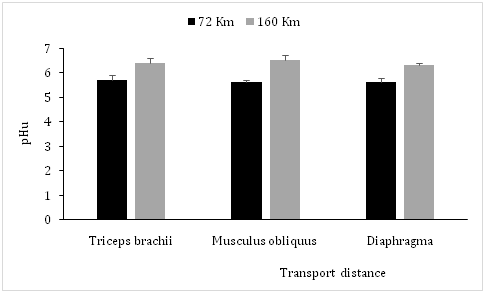
Figure 1 Effects of road transport distance on ultimate pH (24h postmortem) in camel meat. (M±ET, *P<0.05, comparison between long-distance and short-distance).
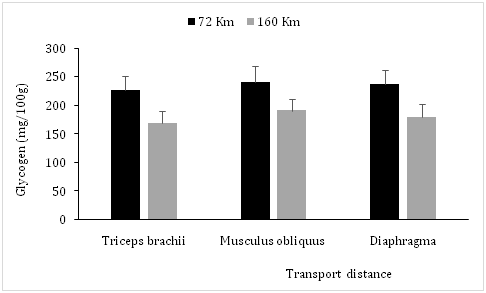
Figure 2 Effects of road transport distance on glycogen in camel meat. (M±ET, *P<0.05, comparison between long-distance and short-distance).
Muscles |
|
Water |
Solids |
Ashes |
Proteins |
Triceps brachii |
72 Km |
73.43±1.45 |
26.57±1.37 |
1.16±0.03 |
19.54±3.54 |
160 Km |
71.35±1.33 |
28.65±1.65 |
1.16±0.03 |
20.13±3.23 |
|
Musculus obliquus |
72 Km |
73.22±1.45 |
26.78±1.68 |
1.15±0.03 |
18.42±2.67 |
160 Km |
70.65±1.41 |
29.35±1.48 |
1.16±0.04 |
19.76±3.13 |
|
Diaphragma |
72 Km |
74.56±1.47 |
25.44±1.64 |
1.16±0.04 |
20.32±3.22 |
160 Km |
72.06±1.37 |
27.94±1.67 |
1.15±0.03 |
18.54±3.65 |
Table 1 Effect of preslaughter transport distance on content of water (%), solids (%) and ashes (%), and proteins (g/100g) in camel meat. (M±ET, no significant difference was observed between long-distance and short-distance)
Muscles |
72 Km |
160 Km |
||
MDA |
Catalase |
MDA |
Catalase |
|
Triceps brachii |
0.124±0.011 |
440±44 |
0.181±0.014* |
337±37* |
Musculus obliquus |
0.133±0.013 |
410±41 |
0.175±0.014* |
326±31* |
Diaphragma |
0.131±0.012 |
432±50 |
0.179±0.011* |
330±33* |
Table 2 Effect of preslaughter transport distance on Malendialdehyde (MDA) levels (µMoles/Kg) and catalase activity (UI/g) in camel meat. (M±ET, *P<0.05 comparison between long-distance and short-distance)
In our work, we studied the effect of distance road transport on certain physicochemical parameters (pHu, moisture, dry matter, ash, protein and glycogen) and oxidative stress biomarkers (MDA and CAT) in meat of camels. The meat of the camel is rich in water compared to that of sheep13 and beef.14 In addition, Elkady & Fahmy15 reported that the camel meat contains more water than the buffalo. This content depends on race, gender, individual, age, health, food, slaughter conditions16 and the amount of water the animal before slaughter.17 The rate of ash in the muscle of our animals is comparable to that reported by Ould El Hadj et al.18 and El Khasmi et al.4 in the same species. According to these authors, the solids content depends on the water content of the meat, which is inversely proportional to the dry matter. In chickens, it was demonstrated that the transportation decreases the water content and increases significantly the proteins of the meat of the animal.19,20
The increased pHu and glycogen depletion observed when the transport distance increases, could be explained by a depletion of muscle glycogen due to emotional and physical stress during transport, reducing the postmortem muscle acidification then causing a high pHu.21-24 Indeed, studies have shown that rabbits which had been transported over a long distance (250km), had a significant high pHu compared with that in rabbits transported over a short distance (30km).25 Furthermore, fasting and loading densities during transport are other stressfull factors resulting in a mobilization of fat and carbohydrate reserves which reduces the preslaughter concentration of glycogen then increases the postpartum pHu in muscle.26 An increase of transportation distance and lairage induces a significant elevation of plasma levels of heat shock protein 70 (HSPA1A), cortisol and glucose in cattle,27 posptpartum pH in meat of turkey,28 chickens29 and pigs30,31 resulting in a significant alteration of meat quality.
In our study, the significant increase of MDA levels and the significant decrease of CAT activity in camel meat when travel distance increases, could indicate a high production of free radicals resulting an OS in muscle. Free radical production would have adverse effects on meat causing its lipid peroxidation and alteration of basic and aromatic aminoacids such as cystein.32,32,33 In ducks34 and pigs35 a transport for 2 hours induced a significant high circulating levels of MDA compared to those observed in non transported animals. In camel, transportation stress can induce an increase of circulating levels of cortisol, lactate, glucose and MDA which is positively correlated with travel distance.4,6 This response may be mediated by an activation of the hypothalamo-hypophyso-adrenal axis during transport stress and may reduce the preslaughter amount of glycogen then affect the postmortem biochemical evolution in muscle. The high impact of road transport distance on the intensity of OS responses has been reported by other research in domestic animals.5,7,8
The high ambiant temperature and loading density during transportation of our camels, may have an amplifying action on the stress responses. Indeed, it has been demonstrated that pigs which were carried at an ambient temperature of 30°C had a significantly higher MDA levels, and an activity of significantly lower catalase compared to pigs having carried at ambient temperature (25°C).36 The same effects of the T° transport on the same biomarkers of OS in muscle were observed in chickens.37,38
As conclusion, the results taken together underline a significant gradual increase of meat pHu and MDA levels and a significant decrease of meat glycogen levels and CAT activity with road transport distance, and could be explained by an antemortem OS activity during travel rising much with distance. Thus, the maximum care should be taken during this situation like the use of vitamins C and E as antioxidants. To confirm our hypothesis, other factors must be taken in consideration such as the use of animals handled differently and the differences of trucks, drivers, days travel and road topographies.
The authors thank the President of urban municipalities of Casablanca and the Director and the responsible for veterinary of Casablanca slaughterhouse, to carry out this work.
The authors have non-financial competing interests in an exclusive academic way.

©2016 Kaoutar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.