MOJ
eISSN: 2381-182X


Research Article Volume 10 Issue 1
Department of Botany, University of São Paulo, Brazil
Correspondence: Antonio Salatino, Department of Botany, Institute of Biosciences, University of São Paulo, Brazil, Tel +5511-997984991
Received: October 07, 2022 | Published: October 25, 2022
Citation: Salatino A. Geopropolis: taxonomic dependence and compositional drawbacks. MOJ Food Process Technols. 2022;10(1):8-10 DOI: 10.15406/mojfpt.2022.10.00266
The contents of total ashes and ethanol soluble substances were determined for propolis samples from species of two Meliponini genera: Melipona and Scaptotrigona. All Melipona samples corresponded to geopropolis (propolis containing aggregated soil material), with ash contents above 40%. Only one species of Scaptotrigona was shown to produce geopropolis, but with ash content (12%) much lower than Melipona samples. In Melipona a high negative correlation was observed between ash and ethanol solubles. Not only aggregated soil may contribute to lower the content of ethanol solubles. It is hypothesized that wax content may have a similar influence.
Keywords: apidae, stingless bees, melipona, scaptotrigona, propolis, phenolic substances
Propolis is a resinous apiculture product used commercially in industries of food and hygiene products, as well as in traditional and popular medicine. Nearly all marketed types of propolis derive from honey bees (Apis mellifera). While bee laborers produce honey from nectar collected from a wide diversity of plant species, they elaborate propolis by mixing resin from a relatively low diversity of plant species, which they mix with beeswax.1,2 Propolis has complex chemical composition. Most propolis types contain predominantly phenolic compounds, a class of secondary metabolites known to exert a wide diversity of biological activities.3,4 Among members of the family Apidae, Apis mellifera is not the only propolis producer species. Many species of stingless bees (Meliponini) also produce propolis, often with composition similar with honey bee propolis.5 Meliponine propolis has been shown to exert a diversity of biological properties,6 including antitumor activity.7 There is a high diversity of meliponines in tropical habitats, with the number of species in the New World reaching nearly 400. They are relevant pollinators of native plants and crops.7 Main meliponine genera in Brazilian biomes are Melipona, Scaptotrigona and Tetragonisca. Comparing with honey bees, the productivity of meliponines is considerably low. For this reason, meliponine propolis is seldom commercialized. However, in recent years there has been a growing interest aiming to produce meliponine honey and propolis. In several parts of Brazil, meliponiculture has been practiced by leisure or desire to contribute with native bee conservation.8 Similar practices have been noticed in Australia.9
Some meliponines produce propolis containing soil material mixed with resin and wax, a reason why this type of propolis is called geopropolis.8 It is not clear the extent among meliponines of the behavior of aggregating soil to propolis. In some papers, it is implied that geopropolis production is generalized among stingless bees.10 Other papers deal with results about geopropolis, but also of propolis of meliponines.11 So far, it is not known if geopropolis is characteristic of species or genera of meliponines, or, alternatively, if the same species may produce propolis and geopropolis. A proper procedure to verify if a meliponine product is propolis or geopropolis is to determine its content of ashes. Ash contents similar with the corresponding parameter of honey bee propolis indicate absence of aggregated soil, hence the material is not geopropolis. The maximum admitted content of ashes in honey bee propolis is 5%.12 Ash contents of several samples of Brazilian green propolis were found to be lower than 4%.13 Ash contents considerably higher than 5% are characteristic of geopropolis. It seems logical that the higher the content of aggregated soil in geopropolis, the lower the content of biologically active substances, such as phenolic substances, since these constituents are soluble in alcohol. The aim of the present work is to obtain a first evaluation of the extent of production of propolis and geopropolis by Brazilian native meliponines. Two main genera of tropical meliponines were selected for this purpose: Melipona and Scaptotrigona. It is aimed also to determine the content of ethanol solubles in all products and verify possible correlations between the two parameters.
Propolis/geopropolis samples
Sixteen samples of propolis or geopropolis were analyzed. They were produced by species of two genera: Melipona and Scaptotrigona. The samples were produced in meliponaries from the Brazilian states Pará (northern Brazil), Maranhão and Rio Grande do Norte (northeast), and Minas Gerais and São Paulo (southeast) (Table 1).
Total ethanol solubles
The procedure by Matsuda13,14 was used, with modifications. Crude propolis was extracted with ethanol for 5 h in Soxhlet. The extract was maintained at -20 °C overnight and filtered through Whatman filter paper n. 1 and evaporated to dryness in flasks previously weighed.
Ashes contents
The samples of crude propolis or geopropolis (3g) were placed in previously weighed crucibles and incinerated over Bunsen burner until final carbonization. The crucibles were transferred to a muffle furnace and maintained at 650 °C for 5 h. After cooling in desiccator at room temperature, the crucibles were weighed.
Statistical analysis
All analyses were performed in triplicates. Means and standard errors were determined. The results were distributed in a scatter plot using Cartesian coordinates. Equation slopes and correlation coefficients (R2) were calculated separately for points relative Melipona and Scaptotrigona.
Highly distinct means were obtained regarding the contents of ashes, comparing the samples of Melipona and Scaptotrigona. Values regarding materials from Scaptotrigona were in the range 1%-12%, while values from Melipona varied from 43% to 81%. However, the range of variation concerning total ethanol solubles was not much different comparing the samples from both genera: Melipona – 1%-46%; Scaptotrigona – 12%-38%. The standard errors corresponding to ash contents were negligible. However, high variation was noted concerning values of ethanol total solubles, even regarding samples of the same species and meliponary (Figure 1). A scatter plot combining the two series of parameters of Melipona and Scaptotrigona (Figure 1) enabled the attainment of regression slopes. The regression equations and correlation coefficients for slopes of the two genera were very distinct: y = 98 – 1.2x and R2 = 0.96 for Melipona, and y = 8 + 2.7x and R2 = 0.64 for Scaptotrigona. The results regarding Melipona indicate that all samples analyzed correspond to geopropolis because their ash contents exceeded by far the upper limit acceptable for honey bee propolis (5%; Brasil 2001). On the other hand, among the samples of Scaptotrigona only S. tubiba (St; meliponary from Guarujá; Table 1), with ash content of 12% (Figure 1) is a geopropolis. All other samples of Scaptotrigona (Sp, Sb1, Sb2, Sx1 and Sx2) contained low amounts of ashes (1% or 2%, Figure 1). Another way to distinguish between propolis and geopropolis is the color of the ashes inside the crucible: ashes derived from calcination of propolis have grey color, while ashes of geopropolis have brown-yellowish color, due to the presence of clay iron salts. Samples from Melipona provided brown-yellowish ashes, while most samples from Scaptotrigona provided grey ashes (Figure 2 A and B, respectively). The results obtained in the present work indicate that most species of Melipona and Scaptotrigona differ in their behavior regarding the production of propolis: the former aggregate soil material or mud to propolis, hence they are geopropolis producers; most species of Scaptotrigona, on the contrary, produce propolis devoid of soil material. Even the unique example in the present work of a Scaptotrigona geopropolis producer (S. tubiba) is distinct from all species of Melipona, if we consider the ashes content obtained: 12% for S. tubiba and 43%-81% for Melipona species (Figure 1).
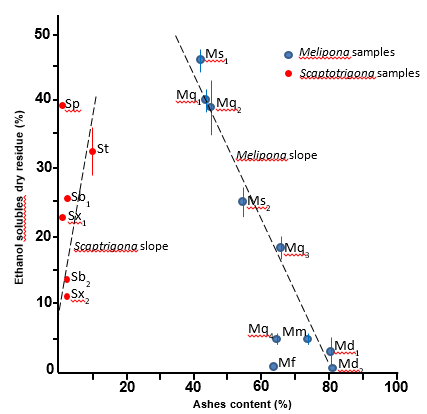
Figure 1 Relationship between contents of total ashes and total ethanol solubles of propolis and geopropolis “from species of Melipona and Scaptotrigona (Meliponini, Apidae)”.
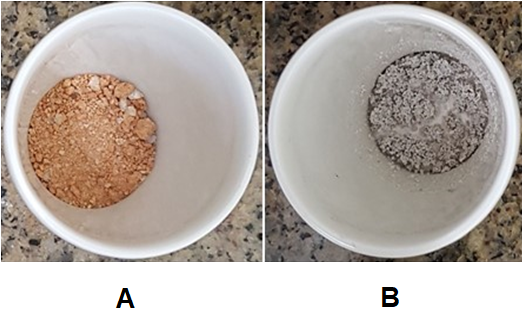
Figure 2 Ashes obtained from calcination of a geopropolis from a species of melipona (A) and a species of scaptotrigona (B; meliponini, apidae).
Because the contents of ashes and ethanol solubles in Melipona are highly correlated (R2 = 0.96; Figure 1), geopropolis with higher contents of soil material contain lower amounts of biologically active substances. As seen in Figure 1, for several samples with ash contents higher than 60% (Md1, Md2, Mf, Mm, Mq3 and Mq4) the contents of ethanol solubles lie in the range of 2%-6%. The lowest officially admitted content of ethanol solubles in propolis of Apis mellifera is 35% (Brasil 2001). In the present work, only four samples complied with this requisite: Mq1, Mq2, Ms1 and Sp (Figure 1). High intraspecific contents of both total ashes and total ethanol solubles were observed for samples of Melipona. For example, the contents of ashes and ethanol solubles were, respectively, 43% and 40% for Mq1, but 18% and 63% for Mq3. Such difference may be attributed to geographic factors, which are known to influence considerably propolis composition.15 Regarding Scaptotrigona, there is virtually no correlation (R2 = 0.64) between the contents of total ashes and ethanol solubles. Although practically no variation was observed in the contents of ashes of samples Sb1, Sb2, Sp, Sx1 and Sx2, their content of ethanol solubles varied from 11% (Sx2) to 38% (Sp; Figure 1). Therefore, other major propolis constituents exert influence, reducing the content of ethanol solubles. A major propolis constituent that may be pointed out in this regard is the wax content. It is suggestive that Sb1 and Sx1, both from a meliponary in the municipality of Guarujá (Table 1) had higher contents of ethanol solubles than Sb2 and Sx2, both samples derived from a meliponary in the municipality of Jacuí (Table 1; Figure 1). Availability of plant sources of resin, which is affected by several factors, including geography16 may account for the observed results.
|
Species |
Codes |
Localities (municipalities/states) |
|
Melipona flavolineata (Friese, 1900) |
Mf |
Castanhal; Pará |
|
M. marginata (Lepeletier, 1836) |
Mm |
Guarujá; São Paulo |
|
M. mondury (Smith, 1863) |
Md1 |
Cotia; São Paulo |
|
M. mondury |
Md1 |
Cotia; São Paulo |
|
M. quadrifasciata (Lepeletier, 1836) |
Mq1 |
Cotia; São Paulo |
|
M. quadrifasciata |
Mq2 |
Cotia; São Paulo |
|
M. quadrifasciata |
Mq3 |
São Paulo; São Paulo |
|
M. quadrifasciata |
Mq4 |
São Paulo; São Paulo |
|
M. subnitida (Ducke, 1910) |
Ms1 |
Mossoró; Rio Grande do Norte |
|
M. subnitida |
Ms2 |
Mossoró; Rio Grande do Norte |
|
Scaptotrigona bipunctata (Lepeletier, 1836) |
Sb1 |
Guarujá; São Paulo |
|
S. bipunctata |
Sb2 |
Jacuí; Minas Gerais |
|
S. postica (Latreille, 1804) |
Sp |
Barra do Corda; Maranhão |
|
S. tubiba (Smith, 1863) |
St |
Guarujá; São Paulo |
|
S. xanthotricha (Moure, 1950) |
Sx1 |
Guarujá; São Paulo |
|
S. xanthotricha |
Sx2 |
Jacuí; Minas Gerais |
Table 1 Species of Meliponini which produced propolis and geopropolis analyzed in the present work, respective codes mentioned in the text, and localities (municipalities and states) of the corresponding meliponaries
The results indicate that the habit of aggregating soil to propolis is taxonomically dependent. Probably most species of Melipona are geopropolis producers, while most Scaptotrigona species produce propolis. A high content of ashes implies that the corresponding geopropolis is a poor vehicle of biologically active substances. However, propolis from meliponine taxa devoid of soil material may also present reduced flavonoids and other phenolic substances, possibly due to high wax content. A proper quality evaluation of meliponine propolis and geopropolis requires the quantitative determination of classes of several chemical constituents.
“The authors thank provision of funds by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, grant 2013/23870-0). AS is Senior Fellow Researcher of CNPq (Conselho Nacional do Desenvolvimento Científico e Tecnológico).
None.

©2022 Salatino, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.