MOJ
eISSN: 2381-182X


Research Article Volume 12 Issue 2
Department of Nutrition and Food Science, University of Dhaka, Bangladesh
Correspondence: Ontara Khatun, Department of Nutrition and Food Science, University of Dhaka, Bangladesh, Tel +8801521488995
Received: September 05, 2024 | Published: September 27, 2024
Citation: Khatun O, Khanam J, Nazrul-Islam S. Effect of functional food and nutraceutical to alleviate oxidative stress in experimental animal. MOJ Food Process Technols. 2024;12(2):156-160. DOI: 10.15406/mojfpt.2024.12.00313
Introduction: Oxidative stress refers to the excessive production of reactive oxygen species (ROS) in the cells and tissues and antioxidant system cannot be able to neutralize them. Imbalance in this protective mechanism can lead to the damage of cellular molecules such as DNA, proteins, and lipids. Antioxidants such as ascorbic acid (vitamin C), carotenoids, polyphenols, thiols, scavenge free radicals. Functional food and Nutraceutical contains antioxidants that inhibit oxidation. The present study was undertaken to explore the burden of oxidative stress present in significant depression and to measure the effect of functional food and nutraceutical to alleviate this oxidative stress.
Methodology: The study was conducted on rat model. A total of thirty Wistar albino rats were included in this study. Total study period was 14 days for experimental model. Disorder was induced by chemical stress technique. Chemical stress induced depression was made by administering chemical stressor (reserpine) for fourteen days. Each model was divided into six groups, where they were administered OFSP2, Carrot, Ceevit (vitamin C), antidepressant (Clomipramine), and placebo. All the animals was scarified on day fifteen. After meticulous dissection the adrenal gland and the brain samples were collected. Then the blood samples were collected for estimation of Malondialdehyde (MDA), Nitric Oxide (NO) and enzyme activity Superoxide Dismutase (SOD). Data analysis was done by SPSS v22 and Microsoft Excel 2013. ANOVA and Post hoc Tukey test were done to analyze the data.
Results: There was statistically significant difference among the groups in case of adrenal gland (p=0.041), percent weight change (p=<0.0001), MDA level (p=<=0.0001), SOD level (p=0.04) and NO level (p=0.034). But there was statistically insignificant difference among the groups in case of brain weight (p=0.44).
Conclusion: From the all parameters, except brain weight it is concluded that functional food and nutraceutical significantly alleviate oxidative stress.
Keywords: functional food, nutraceutical, oxidative stress, antioxidants
Oxidative stress is defined as biochemical imbalance between free radicals and the pro-oxidant/antioxidants level. In this situation production of reactive oxygen species (ROS) exceeds the capacity of naturally-existing antioxidants defense mechanisms.1
In the maintenance of this balance antioxidants of synthetic or natural origin may have a crucial role.2 Depression has been associated with oxidative stress.3
Mitochondrial oxidative processes generate free radicals, which are highly reactive species chemically. When these radicals become in excess or when the antioxidants system gets consumed, Reactive Oxygen Species (ROS) may react with macromolecules of the cell like fatty acid, DNA, protein, etc., thereby causing damage to these macromolecules. The brain, due to its high metabolic rate, is one of the most vulnerable organs to the damaging effects of ROS.4 The adrenal gland is an essential stress responsive organ and after exposure to oxidative stress it can cause hypertrophy in specific regions of the adrenal gland.5
Functional food is the food that not only necessary for living but also a source of mental and physical well-being. Functional food contributes to the prevention and reduction of risk factors for several diseases or to enhance certain physiological functions.6 Whole foods represent the simplest example of functional food. Orange-fleshed sweet potatoes, and carrots, are considered functional foods because of their high contents of physiologically active components (polyphenols, B-carotene, respectively)
A nutraceutical may be defined as a naturally nutrient-rich bioactive or therapeutically active food, or it may be considered as a specific component of a food (such as beta carotene, polyphenols etc.) that apparently provides medicinal or health benefits, including the prevention and treatment of disease.7 Nutraceuticals are marketed in concentrated forms as a single substance or in combination (such as pills, capsules, powders and tinctures etc.). Functional foods and nutraceuticals constitute a great promise to improve health and prevent diseases through their antioxidants properties because they are rich in antioxidants compound.8
The present study was undertaken to explore the burden of oxidative stress present in significant depression and to measure the effect of functional food and nutraceutical to alleviate this oxidative stress. MDA (malondialdehyde), Nitric Oxide (NO), Superoxide Dismutase (SOD) etc. are biochemical markers. These markers were compared in the serum of twenty-five significant depressed rats with five healthy age and sex matched controls. Malondialdehyde (MDA) and Nitric Oxide (NO) are oxidant parameters whereas Superoxide Dismutase (SOD) is antioxidants defense marker.
Study subject
Initial weight ranging between 90-140 grams and age ranging was 50-60 days the experimental study was conducted on thirty (30) healthy Wistar albino rats. In the beginning of the study unhealthy and diseased rats were excluded. The control group for non -stressed rats were matched for age, sex and other exclusion criteria. This study was approved by Ethics Committee, Dean of Biological Sciences, University of Dhaka.
Methods
After selection of rats based on selection criteria they were acclimatized in the animal house for 14 days before intervention. The entire study period remained fourteen (14) days for this experimental model. After acclimatization for 14 days, the rats were divided into groups. The study model has consisted of six (6) groups. Group A was given 25g of boiled orange-fleshed sweet potatoes; group B was given boiled carrot at a dose of 25g. Group C was given nutraceutical Ceevit (vitamin C tablet) at a dose of 25g. An antidepressant drug Clomipramine was given to Group D at a dose of 25g. Group E was negative control and group F was control giving only basal diet. All animals were exposed to stress except group F. Stress was induced by administering reserpine 0.50mg/kg body weight/day, in this chemical stress experimental model.
All the animals were sacrificed on the day 15. The blood samples were centrifuged and the serum was separated from the blood. The separated serum was kept frozen and stored at refrigerated temperature until assayed. After dissection of rats, the brain and adrenal glands of rats were collected, washed, weighted by using an electric balance analyzer. Then the brain and adrenal glands were preserved in formalin. The serum MDA level was assessed by Thiobarbituric acid assay method, the serum SOD level was assessed by Pyrogallol auto-oxidation method and the serum NO level was assessed by Griess reagent method. Average values derived from these methods were used for the analysis.
Malondialdehyde (MDA) level estimation was done by thiobarbituric acid assay method. Here 15 % w/v trichloroacetic acid, 0.375% w/v thiobarbituric acid and 0.25 N hydrochloric acid was used as a reagent (TCA-TBA-HCl). 1.0 ml of biological sample was combined with 2.0 ml of TCA-TBA-HCl and mixed thoroughly. The solution was heated for 15 minutes in a boiling water bath. After cooling, the flocculent precipitate was removed by centrifugation at 1000 g for 10 minutes.
The absorbance of the sample was determined at 535 nm against a blank that contains all the reagents except the biological sample. Serum MDA was measured by using the following formula:
MDA concentration, c = OD/bε
Here,
Extinction coefficient ε = 1.56×100000 /M cm
Width of tube b = 1 cm
OD = Optical density at 535 nm
Zn.Cu.SOD level estimation was done by using auto-oxidation of pyrogallol method. Here Tris-EDTA buffer (pH 8.2) and pyrogallol solution (0.2mM) were used as reagent. Tris-EDTA buffer pH 8.2 was prepared by weighing of 2.85g of Tris and 1.11 g of EDTA-Na2 and dissolved them in 1 liter of deionized water (DW). Pyrogallol solution (0.2mM) was prepared by weighing of 0.252 g of pyrogallol and then dissolved it in a solution of 0.6ml concentrated hydrochloric acid diluted in 1 liter of Deionized water. Spectrophotometer was adjusted to read zero using Tris-EDTA buffer. Control and sample test tubes were prepared then pipetted into test tubes (Table 1).
|
Reagents |
Test (µl) |
Control (µl) |
|
Serum |
25 |
- |
|
Tris buffer |
1500 |
1500 |
|
Deionized water |
- |
25 |
|
Pyrogallol |
500 |
500 |
Table 1 Reagent composition for test and control samples in pyrogallol assay
Absorption was read at the wavelength of 420 nm against Tris-EDTA buffer at zero time and after 1 minute of the addition of pyrogallol.
Serum Zn.Cu.SOD was measured by following formula:
% Inhibition of pyrogallol auto-oxidation = (∆A test)/ (∆A control) ×100%
(Cu-Zn) SOD activity (U/ml) = (% inhibition of pyrogallol auto-oxidation)/ (50%)
3.15.5.3 Nitric Oxide (NO) level estimation
Griess reaction was used to analyze nitrate via its catalytic reduction to nitrate. In this procedure N-(1-naphthyl) ethylenediamine dihydrochloride 25ml of a 0.1% (1 mg/ml) solution, Sulfanilic acid 25ml of a 1% (10mg/ml) solution, phosphoric acid 5%, sodium nitrite 1 ml of 1mM were used as reagents. Griess reagent was produced by mixing equal volumes of N-(1-naphthyl) ethylenediamine dihydrochloride and Sulfanilic acid. 50 µL of Griess reagent, 150 µL of the nitrite containing sample and 1.3 ml of deionized water were mixed. The mixture was incubated for 30 minutes at room temperature. A photometric reference sample was prepared by mixing 50 µL of Griess Reagent and 1.45 mL of deionized water. Absorbance of the nitrite-containing sample was measured at 548 nm relative to the reference sample.
A standard curve of nitrite concentration (x-axis) against absorbance (y-axis) was plotted. Nitrite concentrations corresponding to the absorbance of experimental samples were read from the standard plot.
Statistical analysis
Statistical analyses were performed using SPSS software (22.0 version) and Microsoft Excel 2013. Quantitative data were presented as mean ± SD. ANOVA test was conducted to see the difference between groups and within groups. The Post- hoc Tukey test was conducted, if the differences between groups were significant. Differences were considered statistically significant at p<0.05 for the Post- hoc Tukey test.
Weight of adrenal gland, brain and percentage of body weight change of the six groups of rats are presented in Table 2 with statistically significant difference between the groups regarding adrenal gland and body weight change and in case of brain weight statistically insignificant difference between groups. The biochemical parameters of groups are shown in Table 3. In comparison with other group’s serum MDA, serum NO were significantly higher in control group (p < 0.05) figure 1 & 3. In Orange-fleshed sweet potato group serum SOD was substantially higher (p < 0.001) showed in figure 2. Serum MDA level was considerably lower in functional food groups (Orange-fleshed sweet potatoes; 1.34±0.829 and Carrots; 2.43±0.365nmol/ml) and nutraceutical group (Ceevit) compared to the positive control group Clomipramine that is used as antidepressant drug (2.75±0.625 vs. 3.39±0.287 nmol/ml) and negative control group (2.75±0.625 vs. 8.29±1.073 nmol/ml, p=<0.000). SOD level was significantly higher in functional food groups (OFSP and Carrot) compare to Clomipramine group (3.38±3.13 U/ml;2.24±1.20 U/ml vs. 2.03±0.365 U/ml) but SOD level in nutraceutical group was significantly lower than Clomipramine group (1.97±0.245 U/ml vs. 2.03±0.365 U/ml) and insignificantly higher than negative control group (1.97±0.245 U/ml vs.1.29±0.342 U/ml, p=0.577). Serum NO level was considerably lower in functional food group OFSP compared to the Clomipramine group (0.98±0.113 µM/L vs. 1.17±0.165 µM/L). Functional food group Carrot has slightly higher NO level than that of the Clomipramine group (1.19±0.159 µM/L vs. 1.17±0.165 µM/L) but it was insignificant. Serum NO level in the nutraceutical group was insignificantly higher than that of the Clomipramine group (1.26±0.398 vs. 1.17±0.165 µM/L) and markedly lower than that of the control group (1.26±0.398 µM/L vs. 1.54±0.072 µM/L, p=0.05)
|
Groups |
Weight of adrenal gland (g) |
Weight of brain (g) |
Body weight change (%) |
|
Orange-fleshed sweet potatoes (Mean± SD) |
5.20±1.095 |
1.50±0.060 |
-3.60±3.286 |
|
Carrot (Mean± SD) |
7.40±0.894 |
1.45±0.132 |
-14.80±7.694 |
|
L-Ascorbic acid (Ceevit) (Mean± SD) |
6.40±1.517 |
1.54±0.043 |
29.20±4.604 |
|
Clomipramine (Mean± SD) |
6.80±1.924 |
1.52±0.104 |
25.20±7.155 |
|
Normal control (Mean± SD) |
8.00±1.414 |
1.34±0.059 |
25.40±6.427 |
|
Basal diet (Mean± SD) |
7.80±1.304 |
1.52±0.103 |
35.60±6.542 |
Significance
|
ANOVA |
F (5,24) = 2.776, p=0.041 |
F (5,24) = 0.990, p=0.444 |
F (5,24) = 54.67, p=<0.0001 |
|
Tukey Test |
NC VS OFSP, CT, CVT, p= .042*, .983, .477 |
NC VS OFSP, CT, CVT, p= 0.955, 1.000, 0.675 |
NC VS OFSP, CT, CVT, p=.000*, .000*, .921 |
Table 2 Comparison parameters between the groups according to the adrenal gland, brain and per cent body weight change
|
Groups |
MDA (nmol/ml) |
SOD (U/ml) |
NO (µM/L) |
|
Orange-fleshed sweet potatoes (Mean± SD) |
1.34±0.829 |
3.38±3.131 |
0.98±0.113 |
|
Carrot (Mean± SD) |
2.43±0.365 |
2.24±1.200 |
1.19±0.159 |
|
L-Ascorbic acid (Ceevit) (Mean± SD) |
2.75±0.625 |
1.97±0.245 |
1.26±0.398 |
|
Clomipramine (Mean± SD) |
3.39±0.287 |
2.03±0.365 |
1.17±0.165 |
|
Normal control (Mean± SD) |
8.29±1.073 |
1.29±0.342 |
1.54±0.072 |
|
Basal diet (Mean± SD) |
4.94±1.426 |
2.03±0.809 |
0.94±0.072 |
Significance
|
ANOVA |
F (5, 24) =86.44, p=<0.001 |
F (5, 24) = 2.73, p=0.04 |
F (5, 24) =2.92, p=.034 |
|
Tukey test |
NC VS OFSP, CT, CVT, p=0 .000, 0.000, 0.000* |
NC VS OFSP, CT, CVT, p= .042, .034*, .577 |
NC VS OFSP, CT, CVT, p=.969, .190, 0.05* |
Table 3 Parameters of biochemical changes in different groups of rats in chemical stress model
Here OFSP, orange fleshed sweet potato; CT, carrot; CVT, ceevit; NC, normal control; BD, basal diet; MDA, malondialdehyde; SOD, superoxide dismutase; NO, nitric oxide
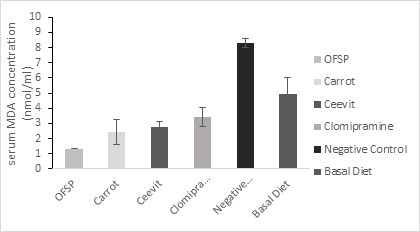
Figure 1 Serum MDA concentration among groups means with the different superscripts are significantly different at P < 0.05 (Tukey’s post hoc test).
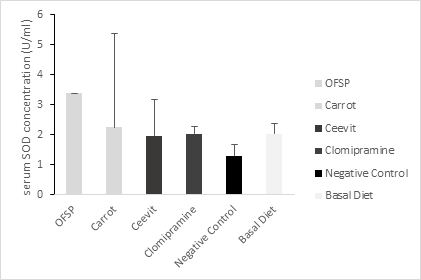
Figure 2 Serum SOD concentration among groups means with the different superscripts are significantly different at P < 0.05 (Tukey’s post hoc test).
In the current study we demonstrated that there was statistically significant difference between functional food group and nutraceutical group in comparison with control group as regards MDA, SOD and NO level. This agrees with what was reported by Del Rio et al,.9 who conducted a study to evaluate MDA was biomarker of oxidative stress. Stress causes production of reactive oxygen species which degrade polyunsaturated fatty acid that raise MDA level.10 However Sahin et al,.11 demonstrated that functional food decrease serum MDA concentration and increase antioxidant status. In galactose‐induced ageing mice nutraceutical, prompts the ability of anti‐ oxidation, anti‐fatigue and anti‐stress, increases SOD activity and decreases MDA level.12
Evidence suggests that various enzymatic and non-enzymatic systems have been developed by the cell to attenuate ROS. However, when a condition of oxidative stress establishes, the defense capacities against ROS becomes insufficient. Therefore, ROS affects the antioxidant defense mechanisms, reduces the intracellular concentration of GSH, decreases the activity of SOD and enhances lipid peroxidation.1 SOD is an antioxidant enzyme capable of reducing superoxide radicals through converting superoxide radicals to H2O2 and H2O.13 In our study, decrease in the antioxidant enzyme activities (SOD) in serum was found in negative control group compare to other groups (p= 0.04). In the present study higher enzymatic activities were found in functional food groups and that are statistically significant (p=0.042 and p=0.034) but in nutraceutical group result is insignificant (p=0.577). In a study reported by Rana et al. patients with abdominal trauma had reduced SOD levels compared with those in the control group.14 In addition, an animal study by Halici et al. showed that SOD levels were reduced in cases of femur fracture.15 However, it has also been reported that SOD levels after trauma are first reduced and then decreased.16
Increased ROS concentrations reduce the amount of bioactive NO by chemical inactivation to form toxic peroxynitrite.17 Peroxynitrite acts as free radicals that can decompose to produce OH. and NO.18 Evidence suggest that in inflammation states NO production by the vasculature increases substantially and in association with ROS, contributes to oxidative stress and in these cases NO play roles in neurodegenerative disorders and serve as neurotoxin.19 In the present study increase NO level in serum was found in control groups compare to other groups (p= 0.034). In functional food groups NO levels were lower but the result is not significant (p= 0.969 and p=0.190) but in nutraceutical the result is significant (p=0.05).
There were significant differences in parameters between the negative control and basal diet group that received no stress which means reserpine produces chemical stress in rat model. Evidence showed that reserpine is a monoamine depletory that exerts a blockade on the vesicular monoamine transporter (VMAT) for neuronal transmission or storage, stimulating dopamine-autoxidation which can increase dopamine levels and oxidative catabolism by monoamine oxidase (MAO).20 The exacerbation of dopamine metabolism in basal ganglia, are rich in monoamines can lead to increase production of free radicals such as highly reactive hydroxyl radicals and auto-oxidation of dopamine into dopamine quinones (which are free radicals in themselves) and superoxide anions which cause neurotoxicity.20
Present study revealed that oxidative stress is associated with some organ change (brain and adrenal gland). The result of this study showed that, weight of adrenal gland in groups OFSP, Carrot, Ceevit was significantly lower than the negative control group and weight of brain was insignificantly higher than the negative control group.
Previous studies have shown that repeated restraint stress alters some physiological phenomenon such as decreased brain weight, adrenal hypertrophy and decreased body weight.21
The brain is more susceptible to oxidative damage when compared to other organs or systems, mainly because it contains high levels of membrane lipids, excitotoxic amino acids, low levels of antioxidant defenses and auto-oxidizable neurotransmitters.20 In this study there was statistically insignificant difference among groups, F (5, 24) = 0.990, p= 0.444. The weight of brain of group OFSP and Ceevit was insignificantly higher than the negative control group (p=0.995, p=0.477 respectively). The mean weight of brain of group OFSP2, Ceevit was almost similar to Clomipramine group.
The adrenal gland is an essential stress responsive organ, several animal studies have shown hypertrophy of specific regions of the adrenal gland after exposure to chronic stress.5 Toxicology studies have demonstrated that hypertrophy can arise from an acute stress response.22 In the study there was statistically significant difference among groups, F (5, 24) = 2.776, p= 0.041. The weight of adrenal gland of group OFSP, Carrot and Ceevit was significantly lower than negative control group (p=0.042, p=0.983 and p=0.047 respectively). The mean weight of adrenal gland of group OFSP, Carrot and Ceevit was almost similar to Clomipramine group.23–26
Functional food groups and nutraceutical group had depleted serum MDA and NO concentration and high level of SOD concentration compared to the negative control group. It suggests that the reserpine induced ROS generation and overuse of exogenous antioxidants available in serum. In conclusion this study showed that functional food and nutraceutical have anti-oxidative stress effect and this could suggest the effective use of Orange fleshed sweet potatoes, Carrots and vitamin C enriched Ceevit have effective role in alleviating oxidative stress in animal.
All the persons involve in this research are kindly acknowledged for their support and special thanks to Professor Dr. Sheikh Nazrul Islam, University of Dhaka. The authors would like to acknowledge Bangladesh Agriculture Research Institute (BARI) and Hamdard Laboratories (WAQF) Bangladesh for financial support.
Authors declare that there is no conflict of interest.
None.

©2024 Khatun, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.