MOJ
eISSN: 2381-182X


Research Article Volume 11 Issue 2
1Federal Institute of Education, Science and Technology of Maranhão, Brazil
2CREA - Research Centre for Food and Nutrition, Rome, Italy
3Federal University of Piauí, Department of Biophysics and Physiology, Brazil
4Federal Institute of Education, Science and Technology of Maranhão, Brazil
5Federal Institute of Education, Science and Technology of Piauí, Brazil
Correspondence: Robson Alves da Silva, Federal Institute of Education, Science and Technology of Piauí, Teresina, Piauí, Brazil, Tel +(86)99405-9537
Received: July 04, 2023 | Published: July 21, 2023
Citation: Borges JM, Lucarini M, Durazzo A, et al. Effect of freezing and freeze-drying on bioactive compounds and antioxidant activity of carnauba pulp (Copernicia prunifera (Mill.) H.E. Moore). MOJ Food Process Technols. 2023;11(2):78-82 DOI: 10.15406/mojfpt.2023.11.00284
Carnauba (Copernicia prunifera (Mill.) H.E. Moore) is a fruit of the Brazilian Caatinga and Cerrado regions. It has good nutritional characteristics, bioactive compounds, and antioxidant activity. However, some processing techniques can affect its chemical characteristics. This study aims at evaluating the effect of freezing and freeze-drying on the bioactive compounds and antioxidant activity of carnauba pulp. The carnauba pulp was submitted to three different treatments: slow freezing (-18 °C/24h), fast freezing (-92 °C/60s) and freeze-drying. Then the bioactive compounds of fresh samples were analyzed - vitamin C, phenolics, flavonoids, anthocyanins, carotenoids - along with the antioxidant activity (DPPH), immediately after processing and after 180 days of freezing storage. It was observed that the process influences the behavior of bioactive compounds and antioxidants, with significant losses of vitamin C, anthocyanins, and carotenoids in all treatments soon after processing, while total phenolic compounds, flavonoids and antioxidant activity increased significantly. At the end of 180 days a reduction of all bioactive compounds and antioxidant activity was observed although the freeze-dried samples presented a better stability of the compounds. Thus, quick freezing and freeze-drying could be representing the more promising technological processes for the maintenance of bioactive compounds and antioxidant activity of carnauba pulp.
Keywords: carnauba, freezing, freeze-drying, bioactive compounds, antioxidant activity
The carnauba palm, also known as "carnaubeira," is a palm tree native to the Caatinga and Cerrado biomes. It is found in the Northeastern (Piauí, Maranhão, Alagoas, Bahia, Ceará, Paraiba, Pernambuco, Rio Grande do Norte, and Sergipe) and Northern (Tocantins) states, but Carnauba populations are concentrated in the states of Piauí, Ceará, and Rio Grande do Norte, always in river valleys and on poorly drained sandy land. The term carnauba derives from the original Indian language tupi and means "scratching tree".1 The palm tree can be used for a variety of purposes, from urban forestry to wax extraction from its leaves, which is used in cosmetics, varnishes, and even for polishing fruit whose focus is on the wax production obtained from the leaves or straw.1,2 Although its fruit is generally used for animal feed, it is also used in human food due to its nutritional value.3 Figure 1 should not only be a way of storing and marketing the fruit for a long period, but it should maintain the amount of the chemical composition found in the fresh fruit; however, studies have shown that processing techniques can drastically affect the chemical characteristics of the fruits.4,5 Thus, the study of the influence of different technological processes (freezing and freeze-drying) on the bioactive compounds and antioxidant activity of carnauba pulp is relevant, as it assesses which losses can be suffered by vegetable raw materials after different processes, as well as whether these changes are significant and detract from the technology used, thus contributing to the expansion of a field of study of this little-explored fruit.
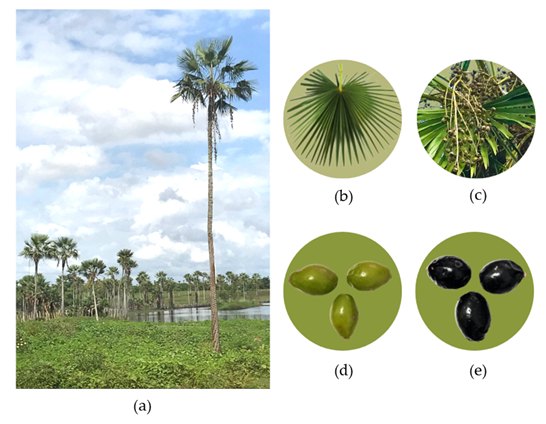
Figure 1 (a) Copernicia prunifera (Mill.) H.E. Moore palm tree; (b) leaf; (c) bunches of fruits; (d) unripe fruit; (e) ripe fruit.
The carnauba fruit has an oval form and resembles miniature coconuts. They congregate in groups of hundreds of units. Unripe fruits are greenish, and as they mature and ripen, they turn purple.1 Bioactive compounds are presented in the fruit, such as phenolic compounds, anthocyanins, carotenoids, flavonoids, and vitamin C.3 These substances intake is important, as they have antioxidant action and help the human body to fight free radicals, thus acting in the prevention of some diseases, contributing to the maintenance of the health of those who consume them.
The chemical composition of the carnauba fruit is an important attraction for consumption, however, as the fruit is available only as fresh product, its consumption only occurs during the harvest period (January to April) and is therefore limited and seasonal. Thus, a viable alternative to make it available all year round is through its processing, such as the pulp production, which can be preserved by freezing or freeze-drying, contributing to better use of the palm tree and income generation, as it adds value to the perishable fruit. It is important to point out that processing should not only be a way of storing and marketing the fruit for a long period, but it should maintain the amount of the chemical composition found in the fresh fruit; however, studies have shown that processing techniques can drastically affect the chemical characteristics of the fruits.4,5 Thus, the study of the influence of different technological processes (freezing and freeze-drying) on the bioactive compounds and antioxidant activity of carnauba pulp is relevant, as it assesses which losses can be suffered by vegetable raw materials after different processes, as well as whether these changes are significant and detract from the technology used, thus contributing to the expansion of a field of study of this little-explored fruit.
Samples
Carnauba fruits were harvested in Campo Maior, located in the state of Piauí. The fruits selection was carried out according to their maturation stage (ripe fruits), visually determined by the dark color of the epicarp, and integrity (appearance, absence of injuries, rot). After harvesting, they were transported in isothermal boxes to the Laboratory of Technology for Products of Vegetal Origin, at the Federal Institute of Piauí (IFPI), Campus Teresina-Central, where they were processed to obtain the pulp. The analyzes of bioactive compounds, antioxidant activity in the Laboratory of Bromatology/IFPI.
Pulp extraction
The carnauba fruits were separated into 3 different batches, and washed to remove surface impurities, rinsed in running water and submerged in a sodium hypochlorite solution at 200 ppm of free chlorine for 20 minutes for sanitization. Then, they were immersed in potable water (5 ppm of free chlorine) for rinsing. The pulp was manually extracted with a previously sanitized stainless-steel knife, from which the edible parts of the fruit (pulp and peel) were removed. They were then homogenized in a processor and packed in 100g polyethylene bags. After bottling, the pulp was subjected to different operations: i) slow freezing; ii) fast freezing and iii) freeze-drying.
Slow freezing was performed in a domestic freezer at -12 °C for 24 hours, while fast freezing was performed in an ultrafreezer, where the pulps were frozen at -92 °C for 60 seconds. For freeze-drying, the pulps were frozen in an ultrafreezer, and placed in a lyophilizer for 72 hours (Figure 2). Samples were analyzed after processing and after 180 days of storage. The pulps frozen by the fast and slow method were kept in a freezer (-12 °C) during storage, and the lyophilized pulp was stored in vacuum packaging at 21 °C and 90% relative humidity (RH), protected with aluminum foil.
Extraction procedures
The extracts for determining the total phenolic content, total flavonoids and antioxidant activity were obtained weighing 2 g of sample into a falcon tube, add 4 mL of 50% methanol. It was homogenized and extracted for 60 minutes in ultrasound at room temperature. The tube was placed in a centrifuge at 4000 rpm for 15 minutes, and the supernatant was transferred to a 10 mL volumetric flask. From the residue of the first extraction, 4 ml of 70% acetone were added, extracted, and centrifuged again, as described above. The second supernatant was transferred to the volumetric flask (10mL) and made up to volume with Milli-Q water.6
Determination of total phenolic compounds
In a 10mL volumetric flask, 2 mL of Milli-Q water was added with 100 μL of the sample (extract). 0.5 mL of Folin-Ciocalteu Reagent was added and vigorously stirred. After 5 minutes, 1.5 mL of 20% m/v sodium carbonate was added. The solution was stirred, and the flask was made up to volume with Milli-Q water. The solution was left to rest for 2 hours and soon after, absorbance readings were taken in a spectrophotometer at 765 nm in an absorbance spectrophotometer. The total phenolic concentration was obtained by interpolating the absorbance on a previously constructed standard gallic acid curve, and the results were expressed in grams of gallic acid equivalent (AGE) per 100 grams of the sample.7
Determination of flavonoids
In a 2mL eppendorf tube, 0.5 mL of the extract and 0.15 mL of 5% NaNO2 were added. After 5 minutes, 0.15 mL of 10% AlCl3 was added. After 6 minutes, 1mL of 1M NaOH was added. After agitation, the absorbance of the sample was read in a spectrophotometer at 425 nm. The result was expressed in mg quercetin equivalents per 100g of sample in comparison with a previously prepared quercetin standard curve.8,9
Determination of in vitro antioxidant activity
Antioxidant activity was determined by the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging method. Initially, a solution of the DPPH radical dissolved in 80% methanol (1:100 v/v) was prepared, adjusting the initial absorbance value (A0) of this solution to 0.800. In test tubes, 100 μL of the sample (extract) were added to 2.9 mL of this solution, and after homogenization, the mixture was kept in a dark place, at room temperature, for 30 minutes. Absorbance measurements were carried out in a spectrophotometer at a wavelength of 515 nm, from the radical, before adding the sample (A0) and after adding the sample, with a 30-minute reaction. A blank test (B) with 2.9 mL of DPPH and 100 µL of solvent was conducted in parallel. A standard curve was constructed with Trolox (6-hydroxy-2, 5, 7, 8-tetramethylchromium-2-carboxylic acid) at different concentrations (0-100 mg/L) as a reference. The results were expressed in μmol TEAC (Trolox Equivalent Antioxidant Capacity) per 100 g of sample.10
Determination of anthocyanins
The total anthocyanin content was determined by the pH difference method. In the initial step, 1.00 g of the sample was weighed and diluted in 10 mL of the solvent Methanol HCl 1.5 N. The diluted sample was then placed in ultrasound for 30 minutes, and subsequently stored in a refrigerator for 12 hours. After resting, 550 µL of the diluted sample was pipetted and transferred to a test tube. 5 ml of potassium chloride solution (0.025 M, pH 1.0) was added, and it was well homogenized, being stored for 10 minutes in the absence of light. The same procedure was repeated for sodium acetate (0.4 M, pH 4.5). The absorbance was measured in a spectrophotometer at the maximum absorption wavelength and at 700 nm. Absorbance was calculated from the following equation: A= (Amax.vis – H700nm) pH1.0 – (Amax.vis – H700nm) pH4.5. The concentration of monomeric pigments in mg/100 g of sample was calculated and expressed in cyanidin-3-glycoside (PM: 449.2 and ε: 26.900) from the equation: (A x PM x FD x 100) / (ε x 1), where: A = Absorbance; MW = Molecular Weight (MW: 449.2); FD = Dilution factor; ε = molar absorptivity (ε: 26,900).11
Determination of carotenoids
The extract was prepared with 5 grams of the sample and 10 mL of acetone: hexane (4:6), placed in an Erlenmeyer flask wrapped with aluminum foil. The mixture was homogenized on a shaking table for 10 minutes. Then, the solution was filtered, and the absorbance read in a spectrophotometer at 450 nm. The result was expressed in mg of β-carotene per 100g of sample in comparison with a previously prepared β-carotene standard curve.12,13
Determination of vitamin C
The vitamin C content was determined using the Tillmans method, which is based on the reduction of 2,6-dichlorophenol indophenol (DFIF) by ascorbic acid.14
Data analysis
For data analysis, the Statistical Package for the Social Sciences (SPPS) software, version 13, was used. The results are presented in tables with the respective means and standard deviations of each studied variable. Variance Analysis (ANOVA) was performed and the Tukey test was applied, adopting a significance level of p<0.05.
The assessment of interaction of bioactive components is the first step to evaluate potential beneficial properties of food matrices.15–18 Generally, the levels of the bioactive molecules could be affected by several factors i.e. genetic factors, cultivar, ripeness, environmental conditions, agronomic practices as well as technologies processing techniques and food preparations.19 In Table 1, phenolic compounds, flavonoids, antioxidant activity, carotenoids, anthocyanins and vitamin C of pulp from fresh carnauba fruit, after processing (slow freezing, fast freezing and freeze-drying), and after the period of 180 days of storage, are reported. Table 1 analysis not performed. Values presented as mean ± standard deviation. GAE: gallic acid equivalent; mgcy-3-Glu: Cyanidin-3-glycoside; mg-β-Carot: mg β-carotene. Small letters compare means, in the same line, between different treatments of the same analysis at the same time and capital letters compare means, in the same column, for the same treatment of the same analysis at different times. Different letters differ significantly, according to Tukey's test at the 5% level of probability.
|
Analyze |
Time (days) |
Treatment |
|||
|
Fresh fruit |
Slow freezing |
Fast freezing |
Freeze-drying |
||
|
Phenolics |
0 |
380.5±0 03c |
437.5±0.02aA |
407.1±0.03bA |
369.1±0.02dA |
|
(mg GAE 100-1) |
180 |
- |
284.41±0.02cB |
333.82 ±0.03bB |
339.54 ±0.02aB |
|
Flavonoids |
0 |
167.9d±0.07c |
179.26±0.01aA |
171.43±0.07bA |
163.0±0.06dA |
|
(mg.100-1) |
180 |
- |
134.28±0.07cB |
140.57 ±0.06bB |
157.75 ±0.04aB |
|
Antioxidant activity (µmol TEAC. 100 g-1) |
0 |
1158.9±0.05c |
1250.2±0.08aA |
1210.4±0.07bA |
1109.1±0,06dA |
|
180 |
- |
987.7±0.06cB |
1040.1 ±0.06bB |
1073.6 ±0.04aB |
|
|
Carotenoids (mg-β-carot.100g-1) |
0 |
2.02±0.02a |
1.89±0.03cA |
1.95±0.02bA |
1.93±0.03bA |
|
180 |
- |
1.03±0.13cB |
1.43 ±0.02bB |
1.54 ±0.02aB |
|
|
Anthocyanins |
0 |
4.31±0.04a |
4.19±0.02bA |
4.24±0.02aA |
4.25±0.02aA |
|
(mgcy-3-glu.100 g-1) |
180 |
- |
4.06±0.03cB |
4.10 ±0.03bB |
4.13±0.02aB |
|
Vitamin C |
0 |
38.1a±0.60a |
35.87±0.01bA |
36.24±0.07bA |
35.03±0.06cA |
|
(mg.100-1) |
180 |
- |
12.9±0.06cB |
22.57 ±0.09bB |
28.04 ±0.08aB |
Table 1 Content of bioactive compounds and antioxidant activity of pulp, from fresh fruits, after processing and after storage for 180 days
The results can be explained by the fact that the ice crystals formed inside the plant matrix can break the cell structure, by allowing the exit of cellular components and access to the solvent, and consequently facilitating the extraction and quantification of phenolic compounds and flavonoids, factor that positively influenced the antioxidant activity soon after processing.20 When comparing the effect of fast freezing and slow freezing on bioactive components, lower values are reported for fast freezing, as in this process the ice crystals form are smaller, by causing less damage to cells, and consequently making the process of extraction and quantification of these compounds more difficult respect to the slow freezing. Since freeze-drying is a more time-consuming process, as it combines freezing and dehydration, the compounds were exposed to adverse conditions for a longer period of time.
Related to the carotenoids content, anthocyanins and vitamin C, the processing contributed to a significant reduction (p<0.05%) of these in relation to the fresh carnauba pulp. Carotenoids are affected by oxidation, exposure to light and oxygen, presence of enzymes, water availability.21,22 Several factors can affect the stability and degradation of anthocyanins such as pH, light, temperature, co-pigmentation, sulfites, vitamin C, oxygen and enzymes.23 Vitamin C can be easily oxidized in contact with water and air.24 Slow freezing facilitates the release of these compounds from the cell matrix that, being more unstable, can end up degrading more easily: this explained the lower values observed for slow freezing respect to quick freezing. Furthermore, when the membranes of cell organelles are damaged during slow freezing, the enzymes they contain are released, such as polyphenol oxide and peroxidase, leading to contact with their substrates, from which they were previously physically separated, initiating enzymatic reactions.24 In the specific case of anthocyanins, the enzyme glycosidase can hydrolyze the ester bonds, causing the release of sugar and aglycone, the latter being unstable and being able to degrade spontaneously, forming the colorless chalcone.25 The blanching process before freezing can be a way to avoid even greater losses of these compounds in the freezing process.26 In this research, the carnauba fruits did not undergo a heat treatment to inactivate the enzymes before freezing, this may have contributed to a greater degradation of these compounds.
Moreover, the interaction between anthocyanins and vitamin C should be also considered. Ascorbic acid can condense with anthocyanins causing the degradation of both ones.27,28 Greater losses in anthocyanin content were not observed in the present study, probably because the vitamin C content in carnauba pulp was not high, so that the chances of anthocyanin losses due to its condensation with ascorbic acid tend to reduce. The data suggest that freeze-drying caused more loss of carotenoids, anthocyanins and vitamin C than slow freezing. Beside it is a more time-consuming process, the reduction of content of bioactive compounds caused by freeze-drying can be related to the time of exposure and manipulation of the samples, in addition to the fact that the dehydration step of this treatment promotes high porosity on the surface of the material, generating increased contact of the product with oxygen.29
Regarding storage, all processes significantly influenced (p<0.05) the number of bioactive compounds and antioxidant activity of the pulp samples during storage for 180 days (Table 1). Although slow freezing causes greater exposure of the compounds, the pulp was not subjected to blanching and, therefore, even stored at low temperature, the enzymatic activity does not stop, facilitating the degradation of these chemical components, causing greater loss of these compounds after the period of storage.30 During the 180 days period, freeze-dried carnauba pulp retained the most bioactive compounds and antioxidant activity during storage. As freeze-dried pulp has a low residual moisture content, this reduces the rates of degradation reactions. Another factor that can influence the retention of bioactive compounds in freeze-dried pulp is the packaging used for storage. Although freeze-drying causes an increase in the contact surface, leaving the food more exposed to the action of external agents, such as oxygen, the use of vacuum packaging wrapped in aluminum foil in this study helped to reduce the contact of the product with oxygen and the light that could accelerate the degradation processes of these compounds, further reducing the antioxidant activity of the pulp.
Slow freezing, fast freezing and freeze-drying process influences the behavior of bioactive compounds and antioxidants of carnauba pulp, with significant losses of vitamin C, anthocyanins, and carotenoids in all treatments, while total phenolic compounds, flavonoids and antioxidant activity increased significantly. At the end of 180 days a reduction of all bioactive compounds and antioxidant activity was observed although the freeze-dried samples presented a better stability of the compounds. Thus, quick freezing and freeze-drying can be represent promising technological processes for the maintenance of bioactive compounds and antioxidant activity of carnauba pulp. Further studies are need in this direction in line with the exploitation of technologies for promotion and valorization of this little-explored fruit.
This study was financed in part by the CAPES e pelo IFPI.
The authors declares that there are no conflicts of interest.

©2023 Borges, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.