MOJ
eISSN: 2381-182X


In the present study changes of bioactive compounds and antioxidant capacity in orange peel treated by pulsed electric fields (PEF), high voltage electrical discharges (HVED) at two equivalent energy inputs (55-364kJ/kg) as well as relative bioaccessibility was evaluated. PEF treatment led to decrease in ascorbic acid when energy input increased. Moreover TPC after PEF and HVED treatments increased significantly with higher energy input applied. By contrast, HVED at 364kJ/kg diminished carotenoids content.
The relative bioaccessibility of carotenoids after HVED (55kJ/kg) was 75.3%. When increasing the energy input from 55 to 364kJ/kg in HVED the bioaccessibility percentage of carotenoids increased in a 82.5%. On the other hand TPC bioaccessibility for PEF at lower energy input was 40.7%. However increasing the energy input to 364kJ/kg led to decrease of the TPC independently of treatment applied. Consequently, results indicated that non thermal treatments such as (PEF, HVED) can enhance the release of bioactive compounds and total antioxidant capacity after in vitro gastrointestinal digestion in orange peel. However, further research is required tooptimize processing conditions.
Keywords: high voltage electrical discharges, pulsed electric fields, bioaccessibility, bioactive compounds, antioxidant activity
Traditionally, food wastes have been considered as a problem. However, they could be a great source of valuable nutraceuticals which can be used to deal with the prospects of feeding fast growing population in 21st century. Perspectives originate from the enormous amounts of food related materials that are discharged worldwide and the existing technologies, which promise the recovery, recycling and sustainability of high-added value ingredients inside food chain.
In this line, a great amount of citric wastes and by-products are generated each year in Europe. Orange peels, which are by-products of orange processing, are a good source of bioactive compounds, such as phenolic content, carotenoids, and vitamin C, which can be used as food additives and/or nutraceuticals.1-4 Several epidemiological studies suggest that bioactive compounds have beneficial effects and have been involved in the reduction of degenerative diseases such as cancers of the lungs and alimentary tract, being this effect mainly attributed to their antioxidant capacity.5,6
Conventional extraction methods used for the recovery of bioactive compounds are based on maceration and heat extraction at temperatures >60ºC alone and/or combined with different solvents, which can be toxic (i.e., hexane, acetone, methanol, etc.). Moreover, the use of high temperatures can promote nutritional losses.7,8 At this stage of development, there is a need to develop new extraction methods that can reduce the extraction time, temperature and solvent consumption and contribute to higher extraction efficiency and lower energy consumption as compared to conventional extraction techniques.
For instance, pulsed electric fields (PEF) and high voltage electrical discharges (HVED) can be useful tools to recover bioactive compounds from fruit by-products. Recent scientific and practical efforts have shown full correspondence of pulsed electric fields (PEF) techniques with green extraction concept.9 This concept assumes using renewable plant resources and alternative solvents (water or agro-solvents (ethanol, methyl esters of fatty acids of vegetable oils)), reduction of energy consumption and unit operations, production of high quality and purity of extracts (non-denatured and biodegradable) and extracts co-products instead of wastes.10 Moreover, methods assisted by pulsed electric energy can allow the increase of the yield and quality of the extracted compounds, thus decreasing the time and temperature of extraction operations.11-17
Some previous studies have evaluated the effectiveness of pulsed electric fields on antioxidant compounds recovery from orange peel15 but there are no studies evaluating the impact of non-thermal treatments (PEF and HVED) upon the bioaccessibility of orange peel thorought a simulated in vitro digestion.
Bioaccessibility has been defined as the fraction of a compound released from the food matrix in the gastrointestinal tract and thus available for intestinal absorption.18 This parameter provides valuable information in order to select the appropriate dosage and source of food matrices as bioactive compounds.
Thus, the objectives of this work were 1) to determine the recovery of bioactive compounds (ascorbic acid, phenolic content and carotenoids) and antioxidant capacity (TEAC and ORAC) from orange peel immediately after pulsed electric fields and high voltage electrical discharges treatments and 2) to evaluate the bioaccessibility of the extracted compounds through a simulated in vitro digestion.
Samples
The orange peels were obtained from orange fruits (Navel lane-late) purchased at a local supermarket (Castellón, Spain) and used immediately.The orange peels were removed from the pulp and chopped into square pieces of 5±1 mm2.
Treatments
A pulsed high voltage power supply (Tomsk Polytechnic University, Tomsk, Russia) that provided 40 kV-10 kA discharges for a few microseconds (≈10µs) was used for electrical treatments. For PEF, the stainless electrodes of the 1-L treatment chamber were two parallel disks. The electrode area was 95 cm2. The distance between the electrodes can be varied from 1 to 10 cm. The circuit configuration and the electrodes shape generated exponential decay pulses. The PEF pulse length was about ti = 10 μs, and the electric field was 13.3 kV/cm. For HVED, the 1-L treatment chamber (inner diameter = 10 cm, wall thickness = 2.5 cm) was equipped with needle-plate geometry electrodes. The diameters of stainless steel needle and the grounded disk electrodes were 10 and 35 mm respectively. The distance between the electrodes was 5 mm. Energy was stored in a set of low-inductance capacitors, which were charged by the high-voltage power supply. The electrical discharges were generated by electrical breakdown in water at the peak pulse voltage (U) of 40 kV. Damped oscillations were thus obtained over a total duration ti of ≈10 μs. The voltage (Ross VD45-8.3-A-K-A, Ross Engineering Corp., Campbell, California, USA) and current (Pearson 3972, Pearson Electronics Inc., Campbell, California, USA) measurement units were connected with a 108 Hz sampling system via an oscilloscope (Tektronix TDS1002, Beaverton, Oregon, USA). The software HPVEE 4.01 (Hewlett-Packard, Palo Alto, USA) was used for data acquisition.
The total treatment duration, tt,(tt = n × tPEF/HVED) was changed by increasing the number of pulses n from 50 to 400. The specific energy input W (kJ/kg) was obtained from Eq. 1:
![]() (1)
(1)
where WPEF/HVED is the pulse energy (kJ/pulse), and m is the product mass (kg). WPEF/HVED is determined from Eq. 2:
![]() (2)
(2)
where U is the voltage (V) and I is the current strength (A), which corresponded to energy inputs of 55 kJ/kg (PEF 1/HVED1) and 364 kJ/kg (PEF 2/HVED2).
Ascorbic content
Ascorbic acid was assayed by polarographic determination using a Metrohm 746 VA Trace Analyzer (Herisau, Switzerland) equipped with a Metrohm 747 VA stand. The working electrode was a Metrohm multi-mode electrode operated in the dropping mercury mode. A platinum wire counter electrode and a saturated calomel reference electrode were used. The following instrumental conditions were applied: DP50, mode DME, drop size 2, drop time 1 s, scan rate 10 mV/s, initial potential −0.10 V. Samples (5 mL) were diluted to 25 mL with the extraction solution (oxalic acid 1% w/v, trichloroacetic acid 2% w/v, sodium sulfate 1% w/v). After vigorous shaking, the solution was filtered through a folded filter (Whatman No. 1). Oxalic acid (9.5 mL) 1% (w/v) and 2 ml of acetic acid/sodium acetate 2 M buffer (pH = 4.8) were added to an aliquot of 0.5 mL of filtrate and the solution was transferred to the polarographic cell. Determinations were carried out by using the peak height and standard addition method in accordance to Carbonell-Capella et al.19
Total phenolic content
Total phenols were determined using a modified method of Georgé et al.20 with some modifications. 10 mL of sample were homogenised with 50 mL of a mixture of acetone/water (7/3, v/v) for 30 min. Mixture supernatants were then recovered by filtration (Whatman® No. 2, England) and constituted the raw extracts (REs). REs (2 mL) were settled on an Oasis cartridge (Waters). Interfering water-soluble components (ascorbic acid and reducing sugars) were recovered with 2 x 2 mL of distilled water. The recovered volume of the washing extract (WE) was carefully measured. In order to eliminate vitamin C, heating was carried out on the washing extract (HWE). All extracts (RE, WE, and HWE) were submitted to the Folin-Ciocalteu method, adapted and optimised.21 Gallic acid calibration standards with concentrations of 0, 100, 300, 500, 700 and 1000 ppm were prepared and 0.1 mL was transferred to borosilicate tubes. 3 mL of sodium carbonate solution (2% w/v) and 100 μL of Folin–Ciocalteau reagent (1:1, v/v) were added to 100 μL of all gallic acid standard and sample tubes. The mixture was incubated for 1 h at room temperature and absorbance was measured at 765 nm.
Total carotenoids
Extraction of total carotenoidwas carried out in accordancewith Lee & Castle.22 An aliquot of sample (2 mL) was homogenized with 5 mL of extracting solvent (hexane/acetone/ethanol, 50:25:25, v/v) and centrifuged for 5 min at 4000 rpm at 5 °C. The top layer of hexane containing the colour was recovered and transferred to a 5-mL volumetric flask and the volume was then adjusted to 25 mL with hexane. Total carotenoid determinationwas carried out on an aliquot of the hexane extract by measuring the absorbance at 450 nm. Total carotenoids were calculated according to Ritter and Purcell23 using an extinction coefficient of β-carotene, E1%=2505.
TEAC assay
The method used was described by Re et al.24 based on the capacity of a sample to inhibit the ABTS radical (ABTS•+) (Sigma-Aldrich, Steinheim, Germany) compared with a reference antioxidant standard (Trolox®) (Sigma-Aldrich, Steinheim, Germany). The radical was generated using 440 μL of potassium persulfate (140 mM). The solution was diluted with ethanol (Baker, Deventer, The Netherlands) until an absorbance of 0.70 was reached at 734 nm. Once the radical was formed, 2 mL of ABTS•+ was mixed with 100 μL of appropriately diluted sample and the absorbance was measured at 734 nm for 20 min in accordance with Carbonell-Capella et al.19 The results, obtained from duplicate analyses, were expressed as: mM TE (millimolar Trolox equivalents).
ORAC assay
The oxygen radical absorbance capacity (ORAC) assay used, with fluorescein as the “fluorescent probe”, was that described by Barba et al.21 The automated ORAC assay was carried out on a Wallac 1420 VICTOR X3 multilabel counter (Perkin-Elmer, USA) with fluorescence filters, for an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The measurements were made in plates with 96 white flat bottom wells (Sero-Wel, BibbySterilin Ltd., Stone, UK). The reaction was performed at 37 °C, as the reaction was started by thermal decomposition of AAPH in 75 mM phosphate buffer (pH 7.0).
Simulated digestion
A three-stage in vitro digestion model was performed based on the previously described procedure by Rodríguez-Roque et al.25 with some modification(the addition of a salivary step). Briefly, 50 mL of each sample(in triplicate) was transferred to an Erlenmeyer flask, and a saliva solution (5 mL, pH 6.75 ± 0.20) containing 2.38 g Na2HPO4, 0.19 g KH2PO4, 8 g NaCl, 100 mg of mucin and α-amylase (200 U/L of enzyme activity) in 1 L of distilled water was added. This mixture was kept in a shaking water bath (37 °C, 90 rpm) for 10 min. Afterwards, 13.08 mg of pepsin from porcine stomach was added and pH was adjusted to 2 by addition of HCl (12 M). This mixture was incubated in darkness in a water bath at 37 °C with continuous stirring (90 rpm) for 2 hours. At the end of the gastric digestion, aliquots were taken for analysis and 20 mL were used for titration with NaOH (0.5 M) to pH 7.5 after adding 5 ml of pancreatin (4 g/L)- bile (25 g/L) mixture.
Dialysis membrane was aconditionated with 0.01 M EDTA Na2, 2% NaHCO3 and 0.1% sodium dodecyl sulfate at boiling point, rinsed with distilled water and cut into segments of 30 cm. Dialysis membrane segments were filled with 25 mL of water-NaHCO3 mixture, with the amount of NaHCO3 (0.5 N) used in the previous titration. 20 mL of the gastric digest were placed into a beaker and the dialysis membrane was immersed in that digest until reaching pH 5.0. This process allows gradual pH adjustment, mimicking intestinal conditions. After 30 min, 5 mL of pancreatin (4 g/L) - bile (25 g/L) mixture was added and the incubation continued for further 2 h (37 °C, 90 rpm). The dialysed intestinal fraction (fraction inside the dialysis membrane), consisting of soluble compounds of low molecular weight, and the non-dialysed intestinal fraction (fraction outside the dialysis membrane), consisting of soluble and insoluble compounds of low and high molecular weight, were collected and placed in a cold water bath for 10 min to stop intestinal digestion. Bioaccessibility (%), referred to the percentage of tested compound remaining in the dialysed intestinal fraction related to the original non-digested sample was determined according to Eq. 3.
![]()
![]() (3)
(3)
Statistical analysis
An analysis of variance (ANOVA) was applied to the results obtained in order to verify whether there were significant differences in the parameters studied in relation to sample analysed, and to ascertain possible interactions between factors (differences at p < 0.05 were considered significant). Where there were differences, an LSD (last significant difference) test was applied to indicate the samples in which differences were observed. A multiple regression analysis was performed to study the influence of bioactive compounds to antioxidant capacity (the results are shown in the significant cases, p < 0.05). Finally, a study was conducted with the aim of determining whether there were correlations between a pair of variables (Pearson´s test). All statistical analyses were performed using Statgraphics® Centurion XVI (Statpoint Technologies Inc., USA).
Changes in bioactive compounds such as ascorbic acid, total carotenoids, total phenolic content as well as antioxidantcapacity due to high voltage electrical discharges (HVED), pulsed electric fields (PEF) of orange peel were studied. To better compare the effects of non-thermal treatments on bioactive compounds stability and bioaccessibility through an in vitro simulated digestion equivalent energy inputs of 55kJ/kg (PEF 1/HVED1) and 364kJ/kg (PEF 2/HVED2) were used.
Undigested samples, immediately after PEF, HVED treatments
Table 1 shows the contents of bioactive compounds and total antioxidant capacity obtained immediately after applying the non-thermal treatments (PEF, HVED) at two equivalent energy inputs (55 and 364 kJ/kg). As it can be observed all factor such as type of treatments as well as energy input had a significant influence in changes, however the extend of improvement ordegradation was different.
The analysis of ascorbic acid showed a noticeable decrease after HVED treatment which can be explained by the formulation of gaseous cavitation bubbles, as well as shocked of waves of high pressure.16 Higher decrease was observed after HVED treatment with higher energy applied, which can be explained by the increase of temperature at high energy levels. Furthermore, we also observed a significant decrease of ascorbic acid after applying PEF treatment with a higher energy input.
On the other hand a significant increase in total phenolic content was found when energy input was augmented for both technologies (364kJ/kg). The highest phenolic content recovery was obtained after applying HVED at 364 kJ/kg. Moreover, the recovery of phenolic content after PEF was significantly lower compared to HVED at equivalent energy inputs. The values obtained for PEF were in the range to those previously reported by Luengo et al.15 who evaluated the effectiveness of PEF on antioxidant compounds applying PEF (1-7 kV/cm) treatments combined with pressing (5-30 min) in orange peel.
PEF1 |
PEF2 |
HVED1 |
HVED2 |
|
AA (mg/100 mL) |
24.6±0.9 |
18.3±0.9 |
14.5±0.9 |
11.9±0.9 |
TPC(mgGAE /100 mL) |
95.8±0.1 |
456.1±0.0 |
184.2±5.5 |
692.1±1.1 |
TC (µg/100 mL) |
- |
- |
369.3±7.1 |
286.9±3.5 |
TEAC (mM TE) |
1.3±0.1 |
3.5±0.6 |
1.2±0.1 |
4.7±0.1 |
ORAC (mM TE) |
1.3±0.0 |
3.5±0.2 |
1.5±0.1 |
5.7±0.1 |
Table 1 Content of bioactive compounds and antioxidant capacity of orange peel after different treatments applied
TPC: Total Phenolic Content; TC: Total Carotenoids; TEAC: Trolox Equivalent Antioxidant Capacity; ORAC: Oxygen Radical Antioxidant Capacity; PEF1: Pulsed Electric Fields (55kJ/kg); PEF2: Pulsed Electric Fields (364 KJ/kg); HVED1: High Voltage Electrical Discharges (55kJ/kg); HVED2: High Voltage Electrical Discharges (364kJ/kg)
Total carotenoids decreased significantly when HVED2 was used at 364 kJ/kg (286.9 µg/100mL) in comparison with HVED 1 (369.3 µg/100mL).This fact can be attributed to the formation of chemical products of electrolysis and free reactive radicals, which are able toreduce nutritional quality of high-added value compounds when HVED is applied at high energy inputs.16,26 Surprisingly PEF treatments caused a total degradation in carotenoids content. A possible explanation for this phenomenon is that PEF does not affect small cell compartments such as chromoplasts, in which carotenoids are mainly found.27
To estimate antioxidant capacity of orange peel two different antioxidant assays; trolox equivalent antioxidant capacity (TEAC) and oxygen radical antioxidant capacity (ORAC) were used. The analysis of antioxidant capacity using both methods (TEAC, ORAC) showed an improvement in antioxidant capacity when higher energy input was applied.
In the case of physicochemical properties, there was no difference observed on pH after different treatments applied (data not shown).
Digestibility of the orange peel extracts treated by two different treatments (PEF, HVED) at equivalent energy inputs
In order to better compare the effects of (PEF and HVED) treatments on bioactive compounds and antioxidant capacity, bioaccesibility thorough an in vitro simulated digestion was analysed. Three-way ANOVA analysis was performed to evaluate the influence of type of treatment, energy input and step of in vitro digestion on changes in content of bioactive compounds and antioxidant capacity. The analysis regarding ascorbic acid content in orange peel treated by HVED and PEF processing during the simulated gastrointestinal digestion is shown in Table 2. During in vitro gastrointestinal digestion, there was a significant decrease in the ascorbic content observed when treatment with lower energy input (55kJ/kg) was applied. After gastric conditions, the recovery of ascorbic acid was in range of 19.5% (PEF1) to 83.3% (HVED2). After the intestinal phase, as well as in the dialyzed fraction, there was no ascorbic acid detected, showing the liability of this vitaminsusceptible to factors such as pH, colour, light and temperature, to which samples are subjected during simulated in vitro gastrointestinal digestion.
Ascorbic Acid (mg/100 mL) |
|
PEF1 |
|
Non-digested |
24.6±0.9a |
Gastric |
4.8±0.9b |
Non-dialysed fraction |
- |
Dialysed fraction |
- |
PEF2 |
|
Non-digested |
18.3±0.9a |
Gastric |
13.2±0.9b |
Non-dialysed fraction |
- |
Dialysed fraction |
- |
HVED1 |
|
Non-digested |
14.5±0.9a |
Gastric |
4.7±0.9b |
Non-dialysed fraction |
- |
Dialysed fraction |
- |
HVED2 |
|
Non-digested |
11.9±0.9a |
Gastric |
9.9±0.9b |
Non-dialysed fraction |
- |
Dialysed fraction |
- |
Table 2 Content of ascorbic acid of orange peel during simulated gastrointestinal digestion after different treatments applied
PEF1: Pulsed Electric Fields (55 kJ/kg); PEF2: Pulsed Electric Fields (364 kJ/kg); HVED1: High Voltage Electrical Discharges (55 kJ/kg); HVED2: High Voltage Electrical Discharges (364 kJ/kg)
Changes in total phenolic content (TPC) during in vitro gastrointestinal digestion shown Figure 1A & 1B.TPC contentwas significantly higher in both cases (PEF, HVED) when energy input was augmented. In addition, the gastric phase revealed non-significant modifications in TPC content after gastric digestion in HVEDcompared to undigested samples. These results are in close agreement to those found by other authors who found that some specific phenolic content from apples appear quite stable after acid hydrolysis in the stomach.28-30 After gastric conditions, the recovery of TPC was in range of 92.7% (PEF 2) to 102% (HVED 1). Regarding intestinal phase, the application of HVED1 led to a difference in TPC of non-dialysed fraction in comparison to undigested sample.Moreover, a significant decrease was found in TPC of non-dialysed fraction when energy input was augmented PEF 2, HVED 2. This fact can be explained by two reasons: some modifications in the specific compounds extracted when energy input was augmented, and/or the formation of hydroxyl radicals during water photodissociation caused by electrical discharges,16 which can reduce the content of these compounds during intestinal phase. The losses which were observed in the non- dialysed intestinal being recovery between 35.5% (HVED 2) to 56.4% (PEF 1) with respect to dialysed fraction.
From our analysis PEF 2 (37.9%) and HVED 2 (35.5%) obtain the lowest recovery compared to non-dialysed fraction. Also Boussetta et al.31 observed a negative effect of electrical discharges when energy values of 80-800 kJ/kg was applied.
On the other hand, as can be observed in Figure 2, it was found a significant increase in carotenoid contentduring gastric digestion and non-dialysed fraction for both HVED treatments compared to non-digested samples. The recovery of carotenoids was for HVED1 (109.3%) and HVED 2 (139%). These results were in close agreement to those reported by Courrad et al. & Rich et al.32,33 they observed that lutein and β -carotene were completely retained or even it was obtained a significant increase in β -carotene at the end of the digestion. These authors attributed this fact to an improved yield promoted by the different processes involved in matrix disruption, thus facilitating the carotenoid extraction from food matrix. In the non-dialysed fraction we observed a stable recovery HVED 1 (60.9%) and HVED 2 (65.1%), respect to dialysed fraction.
For instance, in another study,it was reported that particle size had a significant influence in carotenoid bioaccessibility from carrot- and tomato-derived suspensions, observing an increase in the bioaccessibility of these compounds when particle size was smaller.34 As it was previously reported, HVED is a technology which is based on the fragmentation of food matrix, thus facilitating carotenoid bioaccessibility. Moreover, Moelants et al.34 also attributed the increase in bioaccessibility of carotenoids to interactions between compounds of the complex food matrix.
The antioxidant capacity (TAC)of orange peel after PEF and HVED treatments was measured by two different methods (TEAC, ORAC). Regarding TEAC measurements (Figure 3) a significant increase in intestinal phase (non-dialysed fraction) in PEF1 and HVED1 treated samples was observed compared to undigested samples. After gastric conditions a recovery of total antioxidant capacity measured with TEAC methods was in range (61.7%- 92.3%). In non-dialysed intestinal digesta, recovery was lowest (88%) for HVED2 sample.
Figure 4 shows the contents of the total antioxidant capacity measured with ORAC assay obtained during gastrointestinal digestion after applying two different treatments (PEF, HVED) at two equivalent energy inputs (55 and 364 kJ/kg). It shows a significant enhancement of total antioxidant capacity in non-digested samples when both treatments (PEF and HVED) with higher energy input were applied. However the highest value shows HVED 2. The ANOVA analysis shows a significant increase (p<0.05) in the intestinal tract after simulated digestion when ORAC methods were used. In all treated samples the highest value was detected after treatments with higher energy input applied (364 kJ/kg).
Bioaccessibility of the orange peel treated by two different treatments (PEF, HVED) at equivalent energy inputs.
The relative bioaccessibility of bioactive compounds and antioxidant capacity is shown in Figure 5. The bioaccessibility of carotenoids after HVED 1 was 75.3%. On the other hand when increasing the energy input from 55 to 364 kJ/kg in HVED the bioaccessibility percentage increased in a 82.5%. Similarly Bengtsson et al.35 found that the extraction of β-carotene from plant structure and the bioaccessibility can be enhanced by treatment. Moreover, Palmero et al.36 observed that the intensity of the treatment plays a determined role in terms to obtaining a higher fraction of β-carotene that is available for absorption.
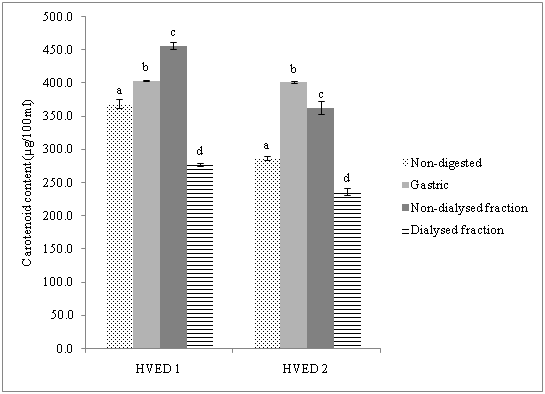
Figure 2 Content of carotenoids during simulated gastrointestinal digestion of orange peel after high voltage electrical discharges.
HVED1: High Voltage Electrical Discharges (55 kJ/kg); HVED2: High Voltage Electrical Discharges (364 kJ/kg)
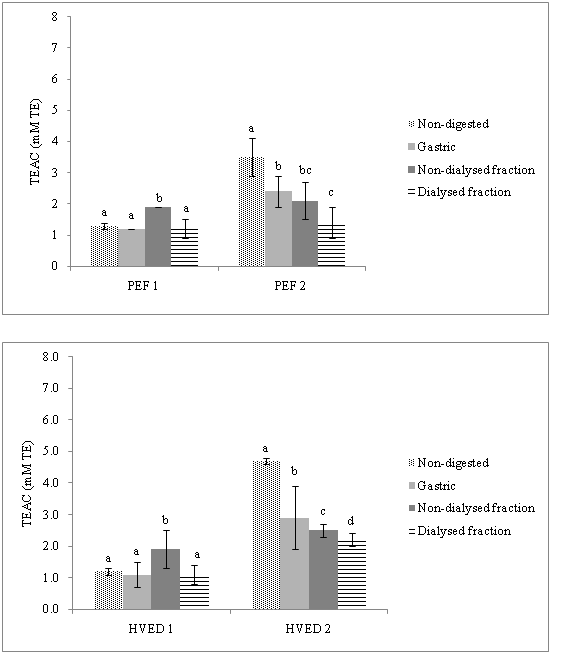
Figure 3 Antioxidant capacity (TEAC values) during simulated gastrointestinal digestion of an orange peel after a) pulsed electric fields (PEF) and b) high voltage electrical discharges (HVED) processing at two energy inputs.
TEAC: Trolox Equivalent Antioxidant Capacity; PEF1: Pulsed Electric Fields (55 kJ/kg); PEF2: Pulsed Electric Fields (364 kJ/kg); HVED1: High Voltage Electrical Discharges (55 kJ/kg); HVED2: High Voltage Electrical Discharges (364 kJ/kg)
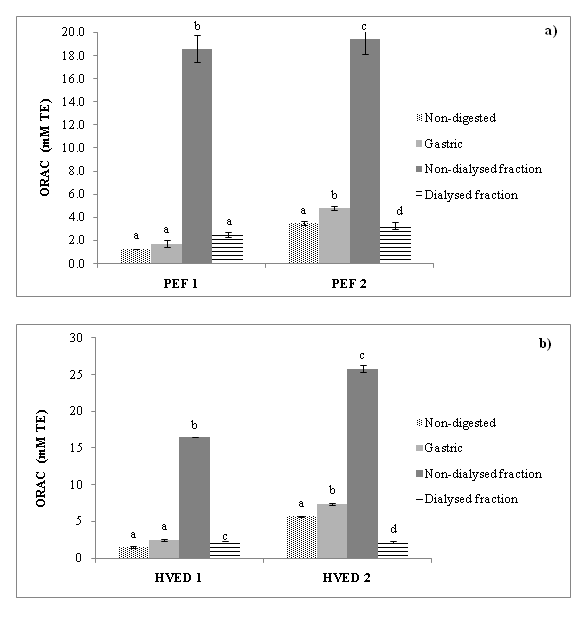
Figure 4 Antioxidant capacity (ORAC) during simulated gastrointestinal digestion of an orange peel after a) pulsed electric fields (PEF) and b) high voltage electrical discharges (HVED) processing at two energy inputs. ORAC: Oxygen Radical Antioxidant Capacity; PEF1: Pulsed Electric Fields (55 kJ/kg); PEF2: Pulsed Electric Fields (364 kJ/kg), HVED1: High Voltage Electrical Discharges (55 kJ/kg), HVED2: High Voltage Electrical Discharges (364 kJ/kg)
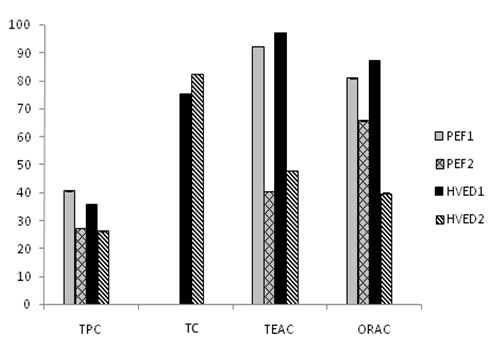
Figure 5 Bioaccessibility of bioactive compounds and antioxidant capacity of orange peel treated by pulsed electric fields (PEF), and by high voltage electrical discharges at two equivalent energy inputs. TPC: Total Phenolic Content; TEAC: Trolox Equivalent Antioxidant Capacity; TC: Total Carotenoids; ORAC: Oxygen Radical Antioxidant Capacity; PEF1: Pulsed Electric Fields (55 kJ/kg), PEF2: Pulsed Electric Fields (364 kJ/kg), HVED1: High Voltage Electrical Discharges (55 kJ/kg), HVED2: High Voltage Electrical Discharges (364 kJ/kg)
Regarding TPC bioaccessibility the highest value was observed when PEF at lower energy input was applied (40.7%). However increasing the energy input to 364 kJ/kg led to decrease of the TPC independently of treatment applied. This phenomenon could be explained by the formation of highly reactive chemicals.
Bioaccessibility of TAC using two different assays (TEAC, ORAC) was also determined. As can be noted the highest bioaccessibility was obtained in the samples treated by HVED1 and PEF1 independently of the method used. By contrast when higher energy input was applied bioaccessibility decreased significantly for PEF 2 (40.5%), HVED 2 (47.9%) according to TEAC method and PEF 2 (65.8%), HVED 2 (39.8%) for ORAC method.
From the results obtained in this study it is possible to conclude that PEF and HVED can be used as a useful tool to recover antioxidant compounds from orange peels. Although HVED led to the higher TPC, carotenoids and TEAC, ORAC yields, PEF treatments at equivalent energy inputs showed the higher TPC and TEAC, ORAC bioaccessibility. So, at this stage of development, there is a need to optimize processing conditions and further studies dealing with this topic are needed.
This research proyect was supported by the Valencian Autonomous Government (Conselleríad´Educació, Cultura I Esport. Generalitat Valenciana) (AICO/2015/083). Buniowska, M. thanks to Podkarpackie Marshals Office for Ph.D. scholarship number (8.2.2/IV.26/217/11/U/155/12) of RSI Project for Podkarpackie Region, Poland. Carbonell-Capella, J.M. thanks the F.P.U. grant (No.AP2010-2546) provided by the Spanish Ministry of Education.Authors thank Dr.E. Vorobiev, Dr.N. Grimi and Dr. F. Barba for their help with preparation of sample treatments from Centre de Researches de Royallieu, Université de Technologie de Compiégne.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.