Journal of
eISSN: 2373-4310


Research Article Volume 6 Issue 5
1M de Vrese, Hamburg, Germany
2G Gerstner, Jungbunzlauer Ladenburg GmbH, Germany
Correspondence: Michael de Vrese, Am Krahenberg 15, D-22587 Hamburg, Germany, Tel +494086642549, Fax +494086642549
Received: September 25, 2016 | Published: June 2, 2017
Citation: Vrese M, Gerstner G. Tricalcium citrate (TCC) and health. J Nutr Health Food Eng. 2017;6(5):130-146. DOI: 10.15406/jnhfe.2017.06.00214
Background: Calcium plays a key role in physiology and bone health. Inadequate intake is common in certain groups of the population.
Methods: Systematic evaluation of relevant literature using medical (Medline) and nutrition databases as well as inclusion of physiological studies, randomized controlled trials, cohort studies, meta-analyses and dietary guidelines
Results and Conclusions: Despite the essentiality of calcium for bone growth and maintenance, clinical studies and meta-analyses on positive effects of calcium supplementation on skeletal health and osteoporosis have shown contradictory results. Dietary recommendations for calcium have therefore been developed primarily in order to meet physiological needs. For individuals who are not able or willing to cover their recommended calcium requirements (1000-1300mg/d for adolescents and adults in industrialized countries) from dietary sources, numerous calcium supplements such as calcium carbonate (CaCO3) or Tricalcium Dicitrate *4H2O (TCC) are available. Of these, TCC is characterized by its good compliance, sufficient calcium content, a better absorbability than CaCO3 and a gastric acid-independent intestinal absorption. Despite the generally poor association between acute effects of high calcium absorption on serum calcium concentration and long term effects on BMD, bone structure and fracture incidence, a higher intake of TCC has led to increased bone mineral density and reduced bone loss in a number of randomized controlled studies in elderly women. Moreover, whereas in some clinical trials and meta-analyzes, (supplemental) calcium caused an increased risk for cardiovascular diseases and stroke as well as for kidney stones, a larger number of studies did not show this or even the opposite, in particular with TCC.
Conclusions: Supplemental TCC is a good opportunity to allow subjects at risk of inadequate calcium supply a calcium intake according to common recommendations. Small positive effects of TCC on bone health have been shown in numerous studies, but the study results are not fully consistent. There is no evidence for an increased cardiovascular disease or renal stone risk.
Physiological role of calcium
The human organism contains about 1200g of calcium, which is the 5th most abundant element in the human body accounting for 1-2% of adult body weight. Almost 99% of calcium is incorporated in the skeleton (0.5% in the teeth), where it brings hardness and also serves as a reservoir for metabolically used calcium. Hardness is provided to vertebrate bones by a form of calcium phosphate, which is close to the composition of hydroxylapatite (Ca10(OH)2(PO4)6) embedded in a protein matrix, mainly collagen fibrils.
Less than 1% (10g in adults) of total calcium is found in the cells of soft tissues and in blood plasma and other extra cellular fluids.1,2 This mobile, metabolically available part of calcium is required for a wide range of essential body functions including intra- and extracellular signaling and nerve impulse transmission, muscle function, vascular contraction and vasodilatation, blood clotting or enzyme activation and mediation of hormonal effects.3,4 Abnormally high serum calcium concentrations (hypercalcemia) may result in lethargy, loss of appetite and/or constipation, sluggish reflexes, and confusion, whereas too low calcium levels may lead to impaired blood coagulation, skipped heart beats, negative effects on muscle contraction and nerve functioning, and–in the long term–brittle bones.
Due to the physiological importance of (low) constant non bone-calcium levels, total and ionized calcium in serum are tightly maintained within a physiological range of 2.1-2.6mM (8.5-10.5mg/dl) or 1.1-1.35mM (4.4-5.4mg/dl), respectively. This is controlled by PTH (parathyroid hormone), vitamin D metabolites (principally calcitriol ((1,25 dihydroxyvitamin D3, 1,25(OH)2D3) and other systemic hormones.
Recommendations for calcium intake
Numerous in vitro and in vivo investigations and clinical studies have undoubtedly proven the essentiality of an adequate calcium supply for bone formation and maintenance as well as maintenance and/or regulation of numerous physiological processes. With this knowledge is an amazing phenomenon that the question of how much calcium is necessary to meet physiological needs and prevent calcium-dependent diseases cannot be definitively answered. Above all, it is still the subject of intense debates whether an increased intake in dietary and/or supplemental calcium above physiological requirements promotes the development of bone mass and reduces the risk of osteoporosis.
In recentyears the recommended daily allowances for calcium have been re-examined. For nutritional purposes, the FAO/WHO,5 the food and nutrition board of the US American Institute of Medicine (IoM 2011)6 and the European Food Safety Authority (EFSA),7 as well as various expert groups from many other countries or groups of countries including the German speaking ones (D-A-CH, Deutschland-Austria-Confoederatio Helvetica),8 have developed sets of Dietary Reference Values (DRVs; Europe)7,9 or Dietary Reference Intakes (DRIs; US),6,10 respectively. DRVs include the Average Requirements (AR) and the Population Reference Intake (PRI), daily intake levels sufficient to meet the nutrient requirements of 50% or 97.5%, respectively, of a healthy population. The corresponding DRI values are the Estimated Average Requirements (EAR) and the Recommended Dietary Allowances (RDA).
Further Dietary Allowances include the Lower Threshold Intake (LTI; intake below which nearly all individuals will be unable to maintain metabolic integrity), the (Tolerable) Upper (Intake) Level (UL, which means the maximum daily intake unlikely to cause adverse health effects) and the Adequate Intake (AI), which is established when evidence is insufficient to develop an RDA and is set at a level assumed to ensure nutritional adequacy.
After reviewing a large number of papers on the relationship between dietary calcium and various skeletal and non-skeletal health outcomes, most expert groups and many medical societies came to the conclusion, that due to inconsistent results of often underpowered and inadequately designed studies, there is insufficient evidence from human trials for calcium health effects that might serve as a basis for deriving calcium requirements.7
Therefore recommendations for calcium intake are mainly based on balance studies or were derived from factorial approaches, i.e. the sum of calcium required for bone accretion, renal calcium excretion, and fecal and insensible (through skin, nails and hair) calcium losses, multiplied with the percentage of intestinal calcium absorption; (Figure 1), rather than on disease-related clinical or surrogate endpoints11 (bone mass, bone mineral density (BMD), bone fractures or osteoporosis).
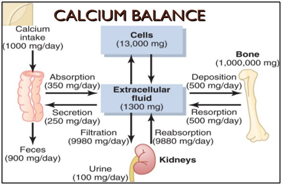
Some medical societies, whose primary interest is prevention or treatment of osteoporosis, justify their (usually higher) recommendations for calcium intake with clinical studies and meta-analyzes, in which an increased calcium intake above the physiological requirements did contribute to increased BMD and/or a reduced risk of osteoporosis.
Table 1 provides as an example calcium recommendations and intake data for Germany, the EU and the USA. The DRIs in the EU,7,12,13 including the German speaking countries (D-A-CH 2015)8 and the DRVs of the US Institute of Medicine (IoM 2011),6 were calculated for growing subjects (children and adolescents from 1 through <19years)) by factorial approaches that sum up calcium accretion (from 90 to 210mg/d) and obligatory calcium losses (between -70 and -290mg/d), multiplied by fractional absorption (46%-38%).14‒18
EFSA Recommendations |
Calcium Intake Germany |
Calcium Intake EU |
US Recommendations |
Calcium Intake USA |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Age |
AR |
PRI |
Age |
Intake |
%Fulfillment of demand4 |
Age |
Intake |
%Fulfillment of demand4 |
Age |
EAR |
RDA |
Intake |
%Fulfillment of demand4 |
|||||
Years |
mg/d |
mg/d |
years |
mg/d |
|
years |
mg/d |
|
years |
mg/d |
mg/d |
years |
mg/d |
|
||||
<1 |
|
280 |
<1 |
♂: 431 |
>>50 |
√√ |
<1 |
♂: 507 |
>>50 |
√√ |
<1 |
|
210- 260 |
|
|
|
|
|
1 - <3 |
390 |
450 |
1 - <3 |
♂: 568 |
>>50 |
√√ |
1 - <3 |
♂: 737 |
>>50 |
√√ |
1 - 3 |
500 |
700 |
<6 |
♂: 809 |
>>50 |
√√ |
|
3-<10 |
680 |
800 |
3 - 10 |
♂: 743 |
>50 |
√ |
3-<10 |
♂: 801 |
±50 |
ÖÖ |
4 - 8 |
800 |
1000 |
6 - 11 |
♂: 843 |
<50 |
√ |
|
10-<18 |
960 |
1150 |
10 - 18 |
♂: 775 |
|
- |
10-<18 |
♂: 868 |
|
- |
9 -18 |
1100 |
1300 |
12 - 19 |
♂: 956 |
|
- |
|
18-<24 |
860 |
1000 |
19-<25 |
♂: 857 |
±50 |
√ |
18-<65 |
♂: 937 |
>50 |
√ |
19 -50 |
800 |
1000 |
20 - 39 |
♂: 856 |
>50 |
√ |
|
≥25 |
750 |
950 |
25-<35 |
♂: 886 |
>>50 |
√ |
65-<75 |
♂: 896 |
>>50 |
√ |
51 -70 |
♂: 800 |
♂: 1000 |
40 - 59 |
♂: 834 |
>50 |
√ |
|
|
|
|
35-<51 |
♂: 838 |
>50 |
√ |
≥75 |
♂: 850 |
>>50 |
√ |
≥70 |
1000 |
1200 |
≥60 |
♂: 716 |
|
- |
|
|
|
|
51-<65 |
♂: 782 |
±50 |
√ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
65- 80 |
♂: 725 |
<50 |
√ |
|
|
|
|
|
|
|
|
|
|
|
|
Table 1 Calcium recommendations and intake data for Germany, the EU (mean of 4 to 8 selected countries) and the USA. Intake data for Germany are from the VELS1 study (children <3 y), the EsKiMo2 study (children and adolescents 3->19 y) and the NVS-24 h recall3 (adults 19–80 y). Tolerable Upper Intake Level (UL) of calcium from all sources (dietary and supplemental) in Europe and Germany are (EFSA 2003): Children and adolescents no recommendations; adults, including pregnant and lactating women 2500 mg/d, the corresponding US-values (IoM 2010) are: infants, children and adolescents 0–0.5 / 0.5-1 / 1-8 / 9–18 years: 1,000 / 1,500 / 2,500 / 3,000 mg/d; adults 19–50 / > 50 y: 2,500 / 2,000 mg/d; pregnant and lactating women < / >19 y: 3,000 / 2500 mg/d
1VELS: Verzehrsstudiezur Ermittlung Der Lebensmittelaufnahme Von Säuglingen Und Kleinkindern21
2EsKiMo: Ernährungsstudieals KiGGS-Modul, Robert-Koch-Institut 2006; KiGGS: Kinder Gesundheits Survey22
3NVS: Nationale Verzehrs Studie II23
4√√ / √ / -: Age Group Good / Satisfactory / Unsatisfactory Supplied With Calcium
For adults (≥19years of age) calcium intake to achieve calcium balance was used, showing zero balance at an average calcium intake of 741mg/day.19 Due to the lack of usable research results for infants <12month, AIs were derived by extrapolating upwards calcium intake data from exclusively breast-fed babies.20 Moreover, in the EFSA recommendations, a PRI of 950mg/day is set for all adults, because the expert group found no clear evidence for beneficial effects of a higher calcium intake on postmenopausal bone loss or the fracture risk in elderly subjects (>65years). By contrast, the IOM committee increased the RDA values for women >50 and men ≥70years to 1200mg/day to reduce bone loss.21‒23
A detailed analysis of the data in Table 1, which takes into account the statistical distribution of individual calcium requirements and calcium absorption shows, that in Germany, the EU and the USA and thus (potentially) generally in developed countries, only infants and children under 3years and perhaps boys between 3 and 10years of age are optimally supplied with calcium. By contrast, calcium supply of ≥50% of the adult population in the EU, of >50% of men aged between 20 and 59, and of less than 50% of 6 to 11years old children in the United States just reaches Average Intake Requirements ((E)AI).
A particular risk for an inadequate supply of calcium exists in times of increased demand (i.e. during skeletal growth), or during age-related reduced nutrient and therefore calcium intake. This means, that children aged between 10 (or 12) and 19years, as well as women over 19 (in the US) or women aged between 19 and 25 and over 65years (in Germany) are groups at risk.
Populations with increased risk also include physically active girls and women participating in sports that emphasize leanness or low body weight (such as ballet, gymnastics and others), a recent meta-analysis showed up to 60% had at least one symptom of the female athlete triad (disordered eating, amenorrhea, and osteoporosis).24 Increased calcium requirements (due to increased bone remodelling) and decreased calcium intake and absorption (resulting from a too low energy and food consumption, and therefore menstrual dysfunction and low estrogens levels) may put the athlete at an increased risk of developing low BMD and osteoporosis, so that to a certain extent, administration of calcium supplements may be helpful, particularly in those athletes with ongoing low consumption of calcium-rich foods.25
Other risk factors for a too low calcium intake include
Nevertheless, it should be kept in mind that an average content of dietary fiber and "ant nutritive" factors and the high percentage of animal protein or phosphate in the "Western-style" diet have already been considered when DVRs/DVIs were calculated.
In the last three decades there has been a substantial expansion of sales of calcium for use as supplements and in food fortification. This also raises the question for the most suitable preparations in terms of price, calcium content, and calcium bioavailability, scientifically proven health effects, side effects and consumer compliance.
Formes of calcium
Several formes of calcium are available: inorganic salts (such as calcium carbonate (CaCO3), calcium phosphate (Ca3(PO4)2)or calcium chloride (CaCl2)) and organic salts with either Ca2+ or calcium (chelate) complexes as cations (e.g. Tricalcium Dicitrate*4H2O (TCC)). There are also calcium supplements from animal or vegetable origin, of which the composition is less well defined, such as milk calcium (comprised mainly of calcium phosphates and caseinates plus small amounts of the citrate), or oyster shell and sea weed calcium (comprised mainly of CaCO3), which however have an at least comparable bioavailability.47 In all classes, there are (practically) insoluble, moderately soluble and highly water-soluble compounds.
However, solubility and bioavailability depend not only on the chemical composition of the calcium compound, but also on its particle size and of the dosage form. For example, in mice "nano" CaCO3 and "nano" TCC (mean particle sizes 151±19/398±4nm) were more bioavailable than the corresponding "micro" compounds (particle sizes 3773±759/1793±382nm),48 whereas administration of calcium supplements in the form of firmly pressed tablets seems to reduce its bioavailability compared to a powdered calcium supplement.49
An overview of the top 5 commercially used and approved calcium salts for use in food, beverages or dietary supplements globally is given in Table 2.50 Of these, CaCO3, TCC and calcium phosphates are the most widely used with a 93% share of products launched recently in dietary supplements and 89% in food and beverages.
Ca Salt |
Ca Content |
Solubility (g/L H2O, RT) |
Main Market Category (Descending Order) |
Share of Products Launched |
|
|---|---|---|---|---|---|
Dietary Supplements1 |
Food & Beverages2 |
||||
Ca Carbonate |
40% |
Insoluble |
Dietary supplements, dairy, baby & toddlers food, cereals |
58.40% |
41.00% |
Ca Citrate (TCC) |
21% |
0.9 |
Dairy, dietary supplements, baby & toddlers food |
20.70% |
11.10% |
Ca Phosphates |
17-36 % |
Slightly soluble to insoluble |
Dairy, baby & toddlers food, dietary supplements, cereals |
14.10% |
36.90% |
Ca Gluconate*H2O |
9% |
3.5 |
Dietary supplements |
2.80% |
0.60% |
Ca Lactate*5H2O |
13% |
66 |
Soft drinks, dairy |
2.40% |
5.90% |
Table 2 Top 5 calcium salts used in dietary supplements and food & beverages and related main market categories.50 Search was based on product launch count 2014-16 globally in the www.innovadatabase.com database on 18.07.2017. To focus on the nutritional use of calcium salts, search was done in the ingredients list of products launched globally in the period 2014-2016 for those products which had “calcium” in the product brand for bone health supplements and which were positioned as “added calcium” for fortified food & beverage products
Compliance of calcium supplements and especially of TCC
Water solubility, absorbability and the concentration of calcium are regarded as the most important quality criteria for calcium supplements in order to meet recommended intakes. In view of positive health effects such as to increase BMD and to reduce risk of osteoporosis and bone fractures, compliance of a calcium preparation is at least as important. This was demonstrated impressively in a meta-analysis of studies on the effect of calcium with or without vitamin D on osteoporotic bone loss and fractures,51 in which fracture risk reduction was significantly greater (24%) in trials in which the compliance rate was high (p<0.0001). Compliance of TCC is thought to be high because of the lack of potential side effects, the neutral taste and the intermediate calcium content, because a high calcium content may on the one hand increase compliance by decreasing the frequency of supplement intake, but on the other hand it increases the risk of side effects and thereby reduces compliance, it impairs calcium absorption and increases potential risks of sharp fluctuations in serum calcium levels.
Intestinal absorption of calcium compounds
In addition to cost, calcium content and compliance, bioavailability of a calcium compound (that means its absorption, retention and adequate use in the body) is the most important quality criterion with respect to supplementation and food fortification. Bioavailability of a calcium compound is often equated with its solubility in water, because calcium is absorbed only in the dissolved state.
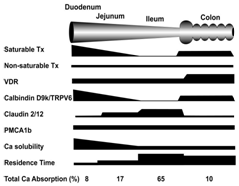
Calcium ions are absorbed in the intestinal mucosa by two distinct routes - a passive, diffusion-driven, and non-saturable transport through the tight junctions between mucosa cells and a vitamin D3-dependend active, transcellular, saturable transport.52‒54 Passive par cellular transport occurs with moderate activity throughout the length of the intestine, driven by the luminal: serosal concentration gradient of Ca2+ ions.
Active transcellular calcium absorption in the intestine is a multistep process, which comprises a) uptake of calcium ions across the apical plasma membrane via the calcium-channel protein TRPV6, b) transcellular transport of Ca2+-ions by calbindin (Ca BP, calcium-binding protein) and c) extrusion of calcium from the enterocytes into the circulation. All steps are controlled by active vitamin D3.55
The saturable calcium transport predominates at low calcium concentrations in the chyme. At high Ca2+-concentrations, however, in segments of the intestine (ileum) with a low efficacy of the saturable component or in neonates and infants (in which this component has not yet been fully developed), calcium absorption occurs predominantly by passive transport. However there are different opinions: while some authors came to the conclusion that at high, but still physiological calcium concentrations intestinal absorption by passive transport exceeds the active component,56 other authors state that under all conditions passive transport plays only a minor role.57
Differential absorption kinetics of calcium from food or from supplements as well as differences between various calcium salts are manifested in earlier and higher rises of serum calcium with supplemental calcium, in a larger total amount of calcium absorbed from food58 and significant differences in the time course of increases in serum calcium and reactive decreases in serum PTH (citrate>carbonate).59
It is frequently claimed that especially due to a relatively high solubility TCC has a better bioavailability than sparingly soluble calcium salts such as CaCO3.
This needs to be scrutinized for several reasons:
Bioavailability of TCC versus CaCO3: results from clinical studies
Absorption and bioavailability of calcium from CaCO3- and TCC-containing supplements have been studied extensively in a number of randomized, controlled clinical trials, using various target parameters and techniques for their determination.
In these studies, between 200 and 1200mg/d of both calcium salts were administered in a cross-over design to healthy men, pre- and postmenopausal women or children, either on an empty stomach or together with a meal.
A meta-analysis of 15 papers published between 1984 and 1999 was performed by.64 In their paper the authors came to the conclusion that calcium absorption is significantly higher by 20% after consumption of TCC than after a CaCO3 supplement containing the same amount of calcium. This value increased to 24% after the authors had eliminated four studies from the analysis due to methodological inadequacies, whereas in the two subgroups, in which the calcium supplements had been administered either with food or on an empty stomach, TCC was better absorbed than the carbonate by 22 or 27%, respectively.
This meta-analysis has sometimes been heavily criticized, because of its great heterogeneity in regard to the study populations, methodology and target parameters. On the other hand, the findings of64 were supported by the fact, that in 9 out of 10 comparable more recent studies published from 1996-2009, of which a large part was put together by,65 TCC showed no poorer and in 7 out of 10 even a better absorbability, regardless of whether they had been measured directly or indirectly by measuring bone parameters (Table 3). In one of these studies, absorption (measured as increase in serum calcium AUC) of TCC was superior to the carbonate only in estrogen-treated patients or in subjects with higher serum 1.25-dihydroxyvitamin D3 concentrations.81 Another study showed superior bioavailability of TCC compared with CaCO3 in obese patients with Roux-en-Y gastric bypass and thereby restricted food intake and impaired calcium absorption.82
Authors (Year) |
N / Study Subjects1 |
Calcium |
Meal |
Method2 |
% Difference |
|
|---|---|---|---|---|---|---|
Metaanalysis by Sakhaee(1999)3 |
||||||
Bo-Linn et al. [65] |
6 n |
1000 |
+ |
L |
21.6 |
|
Nicar et al. [66] |
3 m + 11 w |
1000 |
- |
U |
70.5 |
|
Recker et al. [67] |
7 n |
250 |
- |
S* |
8.0 |
|
Reid et al. [68] |
10 n |
1000 |
+ |
U |
64.8 |
|
Harvey et al. [69] |
9 n |
100/200 |
- |
F |
28.0 |
|
Sheikh et al. [70] |
10 ny |
1000 |
+ |
L |
4.8 |
|
Harvey et al. [71] |
20 w |
500 |
- |
S* |
25.8 |
|
Harvey et al. [72] |
4 m + 17 w |
1000 |
- |
U |
55.7 |
|
Heaney et al. [73] |
17 w |
300 |
+ |
S* |
10.8 |
|
Papers added by van der Velde (2014) |
||||||
Micheletti et al. (1996) [74] |
14 |
1000 |
- |
S* |
Citrate<Carbonate |
|
Heller et al. [75] |
18 wpm |
500 |
+ |
S |
Citrate>Carbonate |
|
Heller et al. [76] |
25 wpm |
500 |
+ |
S, U, P |
46-94 |
|
Heaney et al. [77] |
24 wpm |
200 |
+ |
S, U. P |
Citrate ~Carbonate (ns) |
|
Kenny et al. [78] |
34 w |
1000 |
+ |
U, P, X |
Citrate>Carbonate |
|
Hanzlik et al. [59] |
14 w |
1200 |
- |
S, P |
Citrate>Carbonate |
|
Thomas et al. [79] |
25 wpm |
500/1000 |
- |
P, X |
Citrate>Carbonate |
|
Karp et al. [80] |
12 w |
1000 |
+ |
S, P |
Citrate ~ Carbonate (ns) |
|
Supplemental papers |
||||||
Heller et al. [81] |
25 wpm |
500 |
+ |
S |
242.44 |
|
Tondapu et al. [82] |
18 n5 |
500 |
+ |
S, U, P |
1.9 |
|
Table 3 Studies comparing calcium absorption from calcium citrate (generally in form of Tricalcium citrate (TCC)) compared with calcium carbonate (CC)
1 n/ny: (normal) Subjects/Young Subjects; m/w/wpm : Men/Women/Postmenopausal Women; 2 L: lavage; S/S*: Unlabeled/Labelled Serum Calcium (AUC or peak); F/U: Fecal/Urinary Calcium; P: PTH; X: CTX/NTX bone resorption marker. 3Of the 11 "valid" studies of Sakhaee et al. [64] two further studies were removed in the present table, in which the calcium citrate malate complex had been in used in place of calcium citrate. 4 Only in non-estrogen-treated patients or in subjects with higher serum 1,25-dihydroxyvitamin D3. 5 Obese patients with restricted food intake and impaired calcium absorption due to gastric bypass
Other effects
Furthermore, often mentioned advantages of TCC compared to CaCO3 are the following:
The next two paragraphs cannot be restricted to TCC, because after exposure to gastric acid and pancreatic juice and due to the absorption mechanism, calcium is absorbed in the form of soluble ions, regardless of the administration form. Additional effects of counter ions and/or components of the food matrix must be distinguished from the actual calcium effect. Or in other words, results that were obtained with TCC, provide part of the overall evidence for positive and negative health effects of calcium, while, conversely, results which have been obtained with any (absorbable) calcium compounds, are in general also true for TCC, at least qualitatively.
In addition, most of the studies, in which TCC was used as the supplement, were not specifically designed for the investigation of TCC, but because the citrate is one of the most commonly used calcium salts.
Bone and skeletal health
There is no doubt, that calcium and vitamin D are indispensable for maintaining bone mineral density/content (BMD/BMC) and bone health. There is, however, much debate, whether an adequate or even increased intake of dietary and supplemental calcium with or without vitamin D increases bone mass and bone stability in adolescence and adulthood and thus reduces bone loss and the risk of osteoporosis later in life and particularly in postmenopausal women.
Clinical studies and meta-analyses on the effects of calcium supplementation with or without vitamin D on bone mineral density (BMD) or fractures rates have conflicting results, depending on the duration of the intervention, the observation period, the type of bone and the population group studied: the general population, children, pre- or postmenopausal women, or non-institutionalized or institutionalized elderly. Equally important is the choice of an appropriate study endpoint, because, for example, modest improvements in BMD are not accompanied by reduction of bone fracture rates.85
Whereas in some studies calcium supplements with or without vitamin D were associated with a moderate reduction in bone loss51,86,87 and fractures,51,88 other investigators found no or only marginal differences in fracture rates between subjects taking calcium with or without vitamin D3 or placebo.86,89,90 In a meta-analysis by,85 hip fracture risk was marginally reduced by 8% (RR 0.92) with increased calcium intake from 800 to 1600mg.
In healthy children, calcium has a moderate positive effect on total body BMC and upper limb BMD, being, however, unlikely to reduce fracture risk later in life.91
In elderly (postmenopausal) women with too low calcium consumption, the (longer-term) increase in calcium intake by means of TCC reduced bone resorption and stabilized BMD, possibly by suppressing increased PTH levels. The frequency of fractures was not investigated.92‒95
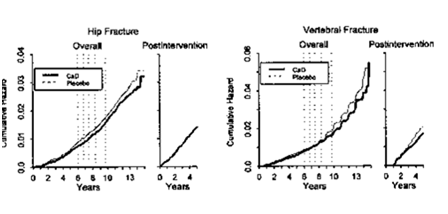
Fracture risk reduction was significantly greater a) in institutionalized subjects over 70years52,96‒98 and with low body weight,52 b) among study participants with a high compliance51 and, because of the slowness of calcium effects on bones - c) in trials where calcium administration was continued and observed over a long period (>5 y). This was demonstrated in the Women's Health Initiative“,98,99 a study in 36282 women, which received 1000mg/d carbonate plus 400 IU/d vitamin D or placebo over seven years (Figure 5).100
Obesity and the metabolic syndrome
In a number of (mainly epidemiological) studies an inverse association between dietary or supplemental calcium and the cluster of metabolic disorders that is termed the metabolic syndrome has been demonstrated, particularly between calcium and obesity,101‒103 insulin resistance,104‒106 hypertension,107 dyslipidemia and obesity-associated low-grade inflammation.108‒110 These effects may be partly explained by increased fecal lipid (and energy) loss and sterol excretion through the formation of poorly soluble fatty acid soaps and bile salts,111 by a reduced spontaneous food intake112 or, particularly with respect to inflammatory complications, by the vitamin D mediated inverse association between calcium intake and intracellular [Ca2+] in adipocytes,113,114 or vascular smooth muscle cells.115 Due to the low number of high-quality studies and participants on this matter, the measured effects are often weak or inconsistent and the underlying mechanisms are poorly understood, so that no intake recommendations can be derived there from.
Kidney stones
Urinary stone disease is characterized by crystalline depositions (calculi), 80% of which are calcium oxalate stones with a variable amount of calcium phosphate. Less than 20% of stones are non-calcium calculi composed out of uric acid, magnesium ammonium phosphate, or cysteine.116 However, the question whether a high intake of calcium from/with food and/or from supplements is associated with an increased risk of chronic hypercalciuria, impaired kidney function, and nephrolithiasis is discussed controversially.117
It could even be that an efficient calcium absorption (e.g. by the use of vitamin D supplements) and thereby large amounts and a high peak concentration of calcium in the circulation increases the risk of calcium oxalate stones. An example for this is the Women's Health Initiative (WHI) CaD supplementation trial, in which daily supplementation with 1000mg calcium plus 400IU vitamin D for 7years was associated with an increase in the incidence of self-reported urinary tract stones by 20% from 2.1% to 2.5% (Table 4).118
Study§ |
Participants$ |
Duration / |
Diets / Intervention+ |
Results# |
Litho-Genicity |
|||
A) Population-Based, Intervention and Prospective Cohort Studies on Total Calcium Intake And Urinary Tract Stones Risk |
||||||||
Different intervent. Studies [122] |
5000 s |
3 mo – 4 y |
8-45 dCa+13-50 sCa |
rUTS± |
± |
|||
HPFS [123] |
45,619 m |
4 y |
max (>26) vs. min (<15)dCa |
rUTSE↓↑t/dCa |
↓ |
|||
HPS [124] |
45,619 m |
14 y |
max(>30) vs. min (<13) dCa |
rUTSE↓↑/±dCa/sCa (m<60y) |
↓± |
|||
NHS I [125] |
91,731 w |
12 y |
max (34) vs. min (13)dCa |
rUTSE↓↑t/dCa |
↓ |
|||
NHS II [126] |
96,245 w |
8 y |
max(>28) vs. min(<16) dCa |
rUTSE↓↑/±dCa/sCa |
↓ |
|||
B) Population-Based Intervention Studies on calcium Plus Vitamin D Supplementation and Urinary Tract Stones Risk |
||||||||
WHI [86] |
|
7 y |
Usual diet (incl. psCa+psVitD) |
isrUTS↑ (HzR 1.17) |
↑ |
|||
WHI [118] |
7 y |
isrUTS↑ (2.1%®2.5%; ns) |
↑ns |
|||||
C) Small Intervention Trials on Calciumcitrate and Urinary Tract Stones Risk |
||||||||
Harvey [127] |
18 s |
⊂⊃ |
20 Ca Citr vs.placebo |
uCa↑; uCitr↑; uOxsat↓; uNP↓ |
↓ |
|||
Levine [128] |
14 sfw |
6 mo |
25 Ca Citr (before-after) |
uCa↑; uCitr↑; uCarbonateOx↓ |
± |
|||
Sakhaee [129] |
7 w |
3 mo |
25 Ca Citr(before-after) |
uCa↑; uP↓; uOx↓; uCitr±; |
± |
|||
Sakhaee [130] |
18 pmw |
4x2 wo;⊂⊃ |
a) placebo; b) 2x10 CaCitr |
vs. placebo: uCa↑; uCitr↑; |
±↓ |
|||
Table 4 Population-based and small intervention studies on calcium ± vitamin D and urinary tract stones risk
§HP(F)S: Health Professional Study (follow-up); NHS: Nurses Health Study (I/II); WHI: Women’s Health Initiative
$S: Subjects; M: Men; W: Women; PM: Postmenopausal; SF: Stone-Forming &MO: Month; Y: Year; ⊂⊃: cross over.
+T/D/SCa: Total/Dietary/Supplemental Ca; Max Vs Min: Highest Vs Lowest Quintile; PS Ca/VitD: personal Ca/VitD suppl.; Ca/KCitr: Ca/Kcitrate
#r/isrUTS: Risc/Incidence of (self-reported) Urinary Tract Stones; ↓↑: inverse association; ±: no association/no effect/no increase; ↑: increase
uCa/uCitr/uP/uOx/uCaOx/uUrAc/uBru: Urinary Calcium/Citrate/Phosphate/Oxalic Acid/Calcium Oxalate/Uric Acid/Brushite(CaHPO4•2H2O);
uOxsat/uBrusat/UNP: Urinary Oxalic Acid Saturation/Brushite Saturation/Nucleation Propensity; HZR: Hazard Ratio; : from...to...; ns: p>0.05.
By contrast, a high intake of calcium alone (and thus a high concentration of calcium in the gut) could reduce the absorption of dietary oxalic acid in the intestine by formation of insoluble calcium oxalate, whereby the stone risk does not increase or could even be lowered (Table 4).
Moreover, using TCC as the supplement may also reduce the risk of renal stones, due mainly to citrate complexation of calcium and to the enhanced citrate excretion which augments the inhibitory activity against calcium oxalate crystallization (Table 4).119
So, the EFSA Panel117 notes that calcium intakes up to about 2400 or 3000mg/d, respectively, have not been associated with an increased risk of impaired kidney function and chronic hypercalciuria, or an increased risk of nephrolithiasis in the adult population. Dietary calcium restriction may lead to bone demineralization and even an increase in stone formation and should not be recommended.120,121
Calcium-alkali syndrome
The term calcium-alkali syndrome (CAS; formerly milk-alkali syndrome) refers to the triad of hypercalcemia, metabolic alkalosis and renal insufficiency, resulting from the long-term ingestion of large amounts of calcium and absorbable alkali (mainly CaCO3).130 Although in individual cases and particularly in the presence of certain risk factors (impaired renal function, metabolic alkalosis) already>1000mg/d calcium may increase the risk of CAS, EFSA117 and IoM6 consider existing Upper Intake Levels (Table 1) as valid.
Calcium and cardiovascular diseases
Epidemiological data, observational studies and randomized controlled trials indicate, that high calcium intake as well as a higher consumption of milk and milk products is inversely associated with cardiovascular risk factors (BMI, (abdominal) obesity, body fat, insulin resistance, LDL-cholesterol and blood pressure)132 and the risk of cardiovascular diseases (CVD), including high pressure and arterial stiffness, coronary heart disease (CHD), myocardial infarction and stroke.133‒136 Even with these results, the contribution of calcium to the overall health effects of milk could not be estimated yet.137
Given these findings, it was surprising when a randomized clinical trial, in which the effect of 1000mg/d TCC on the risk of myocardial infarction, stroke and sudden death has been investigated over 5years in 1471 postmenopausal New Zealand women, found an increased risk of CVD (RR=2.12) with calcium supplements.138 Parts of the results were then confirmed by subsequent evaluation of studies that were not (or not primarily) designed for the investigation of CVD.139‒142 As the cause of atherosclerotic disease, the acute rise in serum calcium was considered, that could be induced with supplemental but not with dietary calcium.
Other authors disagreed with these findings143‒145 with regard to observational studies, meta-analyzes and RCTs, in which no higher cardiovascular risk through calcium with or without vitamin D supplementation has been demonstrated or in which an increased calcium intake was associated even with a reduced cardiovascular risk.146‒148 Indeed, only in six of 28 RCT and observational studies (published between 1992 and 2013), in which effects of calcium on CVD, CHD or stroke as a primary or secondary clinical end point had been examined, an increased risk can be observed, 18 show no effect and four even a lower risk (Table 5).
Study End Point |
Form of Calcium Administration |
Study Design |
N |
Increasd |
No effect |
Lower Risk |
CVD |
Supplements ± Vit D |
RCTs |
6 |
|
5 |
1 (ns) |
CHD |
Supplements+intake |
Observational |
3 |
3 |
|
|
Supplements |
RCTs |
4 |
2 |
2 |
|
|
Intake |
Observational |
6 |
|
5 |
1 |
|
Stroke |
Supplements |
RCT |
1 |
1 |
|
|
Intake |
Observational |
8 |
|
6 |
2 |
|
Total |
28 |
6 |
18 |
4 (1x ns) |
||
And in a recent meta-analysis of 18 randomized controlled studies (with a total of 63,563 participating elderly women) on the effect of calcium on CHD risk (myocardial infarction, angina pectoris, acute coronary syndrome and chronic CHD) and all-cause mortality, the investigators came to the conclusion, that “current evidence does not support the hypothesis that calcium supplementation with or without vitamin D increases coronary heart disease or all-cause mortality risk”.149
In other studies, the increase in serum calcium induced by TCC supplements did not cause a particular high CVD risk, but resulted in changes of vascular parameters that in the long term could reduce that risk.150,151
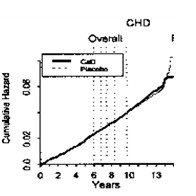
These conflicting study results may be explained, to a certain extent, with methodological weaknesses and also with the slowness of many calcium-dependent processes. For example, where as in the “Women’s Health Initiative” (WHI; see 5.1.) the risk of cardiovascular events through consumption of calcium supplements was not or only very slightly increased during intervention and in the first years thereafter, evaluation of the overall study period even shows a rise of the cumulative hazard in the control group 13years after start of the study99 (Figure 6).
All in all, an increased CHD/CVD risk through higher calcium consumption within consumption recommendations cannot be derived from the available data. Correspondingly, the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) considered in 2012, that long-term calcium intakes from diet and supplements up to 2500-3000mg/day are not associated with an increased risk of cardiovascular disease in adults.117
Mortality
In most studies and meta-analyses, an increased calcium intake (be it from food or from supplements) was not associated with increased mortality due to vascular diseases153‒156 Additionally (total) mortality was even decreased such as in the Iowa Women’s Health Study157 and in the WHI study,158 an increase in mortality hazard ratio with age was more pronounced in the placebo group (Figure 7). In one study,159 mortality showed a U-shaped curve, whereby an intake of dietary plus supplemental calcium between 600 and 1400mg/d was associated with the lowest mortality.
Gastrointestinal Complaints
Calcium carbonate is reported to cause gastrointestinal complaints (upper gastrointestinal gas, bloating or constipation) in sensitive persons,160 which for chemical reasons do not occur with TCC [Section 4.5].
Effects on mineral absorption
Dietary and supplemental calcium at intake levels of current recommendations have been shown to reduce iron and zinc absorption in short term trials. This effect, however, vanished after prolonged continuation of the experiment as well as has not been observed in long-term observational and intervention studies.122,161 TCC is thought to have a smaller or only a marginal negative effect due to the enhancement of mineral and particularly iron absorption by citric acid.162
Calcium and prostate cancer
Several prospective studies and meta-analyses suggest a significantly increased risk of prostate cancer due to increased intake of dietary or supplemental calcium (>1500mg/d),163,164 whereas other large prospective studies165 and meta-analyses166 found no association between dietary and supplemental calcium and prostate cancer or even a protective effect.167 However, it is not clear whether the effect of calcium on prostate carcinogenesis results from the calcium-mediated decrease in 1,25-dihydroxy-vitamin D3, which is thought to have a protective effect against prostate cancer. All in all, available data does not allow for recommendations to decrease or increase calcium intake for cancer prevention.168,169
High calcium intake seems to have a small positive effect on bone mineral content (BMC) and bone mineral density (BMD) in children and postmenopausal women. There is no consistent evidence on the effects of calcium on bone health in premenopausal women or men. Also, the evidence that calcium supplementation reduces fracture incidence is scarce and inconsistent.170
On the other hand, the available data do not provide evidence for an increased risk of kidney stones, cardiovascular diseases and stroke due to higher calcium consumption within intake recommendations.
Therefore, it is not recommended by the respective authorities to change existing intake recommendations or to reduce calcium consumption. These recommendations were not designed to reduce the risk of fractures and osteoporosis and other adverse health effects, but to cover the physiological calcium requirements of certain population groups. This is primarily because of an insufficient quality and contradictory results of the respective clinical studies and meta-analyses.
Therefore the usually quite high intake recommendations for calcium in industrialized "Western" countries is a result from increased calcium requirements in a "Western style" diet, which, inter alias, are due to its high content of (animal) protein. An increased protein intake is not a risk per se, and may even promote bone growth and bone health, when at the same time adequate calcium intake is guaranteed.
A particular risk for inadequate calcium supply exists during skeletal growth and age-related reduced nutrient and therefore calcium intake. At risk groups include: children and adolescents between 10 and 19years; women over 19 (in the US) or women aged between 19 and 25 and over 65years (in the EU); very old, and frequently hospitalized and malnourished people; and lean and physically active (ballet, gymnastic) girls and women.
It is recommended by the respective nutrition societies to meet calcium intake needs by eating calcium-rich foods (in western countries mainly milk and milk products), because calcium administration within a meal is thought to delay calcium absorption and thereby to reduce peak concentrations in serum calcium and the risk of vascular calcification and other side effects. With a "Western style" diet and a calcium absorption rate of 25-30%, daily consumption of 4 servings of milk or dairy products (e.g. 250g milk and yogurt or 1-2 slices of hard cheese per serving) or other calcium-rich foods would be necessary to meet recommended calcium requirements of 1000-1200mg/d for adolescents and adults.
However, it is recognized that many people are not able or willing to meet these requirements through foods naturally containing calcium. For these people, calcium-fortified food or a wide range of calcium supplements (with or without added vitamin D) is available. CaCO3 and TCC are among the compounds most frequently used for this purpose.
TCC has an intermediate calcium content (21%) and, because of the lack of potential side effects and the neutral taste, a good compliance. Solubility of TCC is pH dependent; it increases with decreasing pH, and exceeds that of CaCO3 above pH 6.6. This means that TCC is more soluble than CaCO3 from the middle of the jejunum to the colon The amount of absorbable free Ca2+-ions in the chyme depends on the solubility of the respective calcium salt, the presence of complexing agents and an adequate production of gastric juice, by which also less soluble calcium compounds like CaCO3 or TCC are converted into easily soluble CaCl2 or better said into Ca2+- and Cl- ions.
Due to the irreversibility of Trans- and par cellular calcium absorption, poorly soluble calcium compounds and calcium complexes may also provide sufficient calcium ions for absorption. Therefore absorption and bioavailability of TCC compared to CaCO3 cannot be derived by theoretical considerations but has been examined in a number of randomized, controlled human trials and by a meta-analysis, demonstrating a significantly higher bioavailability (about +24%) of TCC compared to CaCO3.
The association between acute effects of high calcium absorption on serum calcium concentration and long term effects on BMD, bone structure and fracture incidence is generally poor. Nevertheless, a higher intake of TCC has led to increased bone mineral density (BMD) and reduced bone loss and serum PTH in a number of randomized controlled studies in elderly pre- and postmenopausal women. One of the reasons for this may be the good bioavailability and the sufficient calcium content of TCC.
Other, often mentioned advantages of TCC compared to CaCO3 are the following:
The authors declare that they have no competing interests. The Co-author, Gerhard Gerstner, is Business Development Director Health & Nutrition at Jungbunzlauer Ladenburg GmbH, Germany, which, among other products manufacture organic calcium salts, including TCC.
Publication was financially supported by Jungbunzlauer Ladenburg GmbH, Germany.

©2017 Vrese, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.