Journal of
eISSN: 2373-4310


Research Article Volume 4 Issue 2
1Sun Yat-Sen University, China
2Zhuhui Maternal and Child Health Care Center, China
3Enzymotec Ltd, Israel
Correspondence: Prof. Yu-ming Chen, PhD, MD, Department of Medical Statistics & Epidemiology, School of Public Health, Sun Yat-Sen University, Guangzhou 510080, China, Tel +86-20-873-33166, Fax +86-20-873-331-66
Co-correspondence: Prof. Yi-xiang Su, MD, MS, Department of Nutrition, School of Public Health, Sun Yat-Sen University, Guangzhou 510080, China, Tel +86-2087333166, Fax +862087333166
Received: October 25, 2015 | Published: March 15, 2016
Citation: Zhong W, Xin-yi T, Hong-ying H, et al. Tolerance and efficacy of infant formula with high sn-2 palmitate in formula-fed chinese term infants: an open label, controlled trial. J Nutr Health Food Eng. 2016;4(2):379-387. DOI: 10.15406/jnhfe.2016.04.00122
Objectives: We assessed the efficacy of high sn-2 palmitate infant formula as compared with breast-milk in healthy full-term Chinese infants.
Methods: Seventy five term infants, aged 14days or less, appropriate for gestational age (34 formula-fed and 41 breast-fed) were recruited to this open-label controlled multi-center trial. Formula fed infants were assigned to receive a formula with high sn-2-palmitate (40% of the palmitic acid is esterified to the sn-2 position of the triglyceride).
Results: Anthropometric measurements of growth were done at randomization, and at 6 and 12weeks postnatal age. Three-day records were completed by parents on stool characteristics and infants’ wellbeing; face-to-face interviews were conducted according to a structured questionnaire. At 6 and 12weeks of age, all infants presented normal growth parameters. Hard stools were not reported in both groups; however, the FF infants had less stools per day and a lower stool consistency score. The formula fed infants cried longer compared to the breastfed infants. There were no significant differences between the two groups in adverse effects.
Conclusions: The formula containing high sn-2 palmitate had similar effect to breastfeeding on body growth, prevention of hard stools, and maintaining wellbeing in Chinese term infants. However, compared with breastfed infants, the FF group stool in the early 3 months of life was less soft (ClinicalTrials.gov Identifier: NCT01157390).
Keywords: sn-2 palmitate, infant formula, breast milk, body growth, head circumference, stool characteristics, infant crying
In human breast milk and in most infant formulas about 50% of the dietary calories are supplied to newborns as fat, with more than 98% of this milk fat being in the form of triglycerides. The high content of saturated fatty acids in breast milk is easily absorbed by infants. The majority of those saturated fatty acids is palmitic acid (C16:0), which constitutes 17% to 25% of the fatty acids in mature human milk interestingly, 70% to 75% of the palmitic acid are esterified to the sn-2 (ß) position of the triglyceride.1 This preferential positioning is highly conserved in all women, regardless of their ethnic origin or nutrition and is probably responsible for the easy absorption of the highly saturated fatty acids in human milk.2 Palmitic acid was shown to be absorbed from human milk as sn-2 monoacylglycerol3 and is conserved as such through digestion, absorption, and chylomicron triacylglycerol synthesis.4 In contrast, palmitic acid present in vegetable oils, which are commonly used in most infant formulas, is esterified to the sn-1 and sn-3 positions5 and hence less easily absorbed through the gastrointestinal tract of infants. The main cause for this low absorption is the high tendency of free palmitic acid to create complexes with dietary minerals, such as calcium,6 resulting in loss of both calcium and long chain saturated fatty acids, mainly palmitic acid. These complexes, also known as fatty acid soaps or saponified fatty acids, are insoluble and therefore indigestible and are associated with harder stools.7
The use of formula containing triglycerides, with a positional fatty acid profile similar to that of human milk, was shown to be associated with an improvement in the absorption of palmitic acid and better mineral balance in term infants,8‒10 premature newborn infants11 and animal models.2,12,13 Compared with standard formula, fat absorption in formula fed (FF) infant with high sn-2 palmitate acid was higher and the fecal calcium excretion was significantly lower.8 Moreover, it was demonstrated in both preterm and term infants that by increasing the proportion of sn-2 position palmitate in the formula not only that the excretion of palmitate and associated calcium soaps in the stool is reduced and calcium absorption subsequently increased, but also stool hardness is reduced.14 During the last years, the importance of a fat structure which is similar to that of human milk fat, was further revealed by additional benefits known for human milk, that were demonstrated for infant formula with high sn-2 palmitate, such as increasing beneficial gut flora15,16 and reducing infant crying.17 According to our knowledge, this is the first study examining an infant formula with milk fat, prebiotics and structured lipids. The aim of this study was to examine the effect of SN-2 palmitate infant formula in Chinese infants compared to breastfed infants.
Study design and participants
The study was designed as a multi-center, open-label controlled, 12weeks study. Healthy term infants, appropriate for gestation age (AGA) with normal birth weight (2500-4000g) were eligible for the study. The recruitment process was carried out by qualified pediatricians in local hospitals or maternal and Child Health Care centres located in the districts of Guangzhou and Hengyang, China. The infants were screened, using a standardized structured questionnaire, and had a physical examination by a qualified medical team. Exclusion criteria included infants with a congenital abnormality, suspected chromosomal or metabolic disorder or infants receiving medications at the first week of life. Infants of mothers with a health condition or socioeconomic problems that may interfere with their ability to take care of their infants were also excluded. The recruitment of formula fed infants (FF group) followed a maternal unequivocal decision not to breastfeed; the recruitment of breastfed infants (BF group) followed a maternal commitment to breastfeeding for at least 12weeks. In this open-label, controlled, multi-center trial, we used per-protocol analysis, examining only infants who completed the 3-month follow-up.
The FF infants were fed using milk-based commercial formulas produced by Wondersun Co., Harbin, China. The fat fraction component of the formula was composed of structured triglycerides (INFAT® Advanced Lipids AB), consisting 21.9% palmitic acid of total fatty acids of which 41% of the palmitic acid esterified at thesn-2 position of the triglyceride (Table 1). The BF infants were fed only with human breast milk. Except for vitamins A and D, no other supplemental foods were allowed during the study period.
|
FF Infants |
BF Infants |
p value |
Infant characteristics |
|||
Total gestation, d |
277±5 |
274±7 |
0.112 |
Gender, M/F |
18/12 |
16/15 |
0.510 |
Birth weight, g |
3335±339 |
3331±355 |
0.843 |
Birth length, cm |
49±3 |
49±2 |
0.921 |
Maternal characteristics |
|||
Age, y |
27.0±4.4 |
26.0±4.2 |
0.354 |
Primigravida (%) |
36.7 |
48.4 |
0.355 |
Type of delivery (vaginal/caesarean) |
10/20 |
6/25 |
0.215 |
Education (>12 years, %) |
10 |
35.5 |
0.018 |
Table 1 Infant and maternal characteristics
The protocol for this research project was approved by the Ethics Committee of the School of Public Health, Sun Yat-Sen University, Guangzhou, China, conforming to the provisions of the Declaration of Helsinki in 1995 (revised in Edinburgh, 2000). All parents or legal guardians signed a written informed consent form before enrolment; parents/infants anonymity was preserved.
Anthropometric measurements: Infants’ birth weight and length were obtained from hospital records. Growth parameters were assessed at enrolment and at 6 and 12weeks postnatal age by trained technicians. The measurements covered the following variables: body weight (the mean of 3 measurements); body length (the mean of 2 measurements of recumbent crown-heel length to the nearest 0.1cm), and fronto-occipital head circumference (standard 1 cm wide measuring tape to the nearest 0.1cm).
The scores calculated at baseline and at 6 and 12weeks of age for weight, length, and HC were compared to the WHO criteria for infant growth.18
Parents' questionnaires: Data on stool characteristics were collected prospectively by the parents, using 24hour behavior diaries for 3days before the 6weeks and 12weeks visits. The reports included: volume of formula feeding, stool characteristics including color (yellow, green, brown and black), volume (small, normal, or large using sample picture scale), and consistency (scores of 1-6 using sample pictures. In addition, parents filled out data on the infants’ general health and well-being, behavior, and habits including crying time per day, concomitant medication use, and adverse effects.
Statistical analysis
SPSS for Windows (release 17, SPSS Inc., Chicago, USA) was used for statistical analysis. T-test was used for comparisons of calculated means for continuous variables that followed a normal distribution (e.g., weight, height, age). Analysis of covariance (ANCOVA) was used to compare the adjusted means of primary outcomes (weight, height, HC) between the two groups. Variance analysis for repeated measures was used to test differences in growth between the two groups (interaction for time (stage) ´ intervention). Comparison of means was performed after adjustment for potential confounders that were found to significantly affect the statistical model such as gestational age, mother’s age, delivery number, maternal education, second hand smoking, gender, baseline values of the anthropometrics, and intervention duration. The Wilcoxon-Mann-Whitney test was used to compare the difference in the rank sum for continuous dependent variables with a skewed distribution or ordinal variables. For categorical variables, a chi-square test (or Fisher exact test if cells were <5) was used to compare the frequency between the two groups. If the categorical variables were ordinal, the Spearman correlation measured the correlation coefficients between two ordinal variables. The pairwise analyses of crying duration and number of crying episodes were performed using a generalized linear model with Negative binomial distribution and log link. A two-sided p value of less than 0.05 was considered statistically significant.
Seventy five healthy full-term (gestationalage 37 to 40weeks) Chinese infants, aged 14days or less, were initially enrolled to the study. The formula-fed (FF) group comprised of 34 FF exclusive formula-fed infants and the breastfed (BF) group comprised of 41 exclusive breastfed infants.Fourteen infants (4 FF and 10 BF) were excluded due to protocol violation or were lost to follow-up (Figure 1). Sixty one infants (30 FF and 31 BF) completed the study with good compliance (>80%), and were included in the statistical analysis.
Maternal and infant characteristics
Mothers and infant's characteristics are listed in Table 1. Breastfeeding mothers had a significantly higher education level compared with the FF mothers (Table 2). All other maternal characteristics were comparable.
Per 100 Grams Powder |
|
Energy (KJ) |
2115 |
Protein (lactalbumin/casein, 60/40) (g) |
13.5 |
Carbohydrate (g) |
56.1 |
Fat (g) |
25.2 |
Milk fat |
7.5 |
FOS (mg) |
464 |
Percent by total fatty acids |
|
12:0 |
8.6 |
14:0 |
6.15 |
16:0 |
21.9 |
18:0 |
6.3 |
18:1 n-9 |
35.7 |
18:2 n-6 |
13.1 |
18:3 n-3 |
1.5 |
20:4 |
0.51 |
22:6 |
0.33 |
Other fatty acids |
11 |
Ratio of 16:0 in the sn-2 position† |
41 |
Table 2 Composition of Study Formula
†Ratio of 16:0 on the sn-2 position of total 16:0 is normalized per position.
‡Comprises vegetable oil mix of palm kernel oil, rapeseed oil, sunflower oil, and structured triglycerides.
Additionally, no significant differences were observed between the groups for infant characteristics but the inclusion age which was higher in the FF group.
Anthropometric measurements
Anthropometric measurements are summarized in Table 3. Some differences were observed at baseline due to the differences in the age at inclusion. No significant differences were observed in any anthropometric parameter following adjustment for the relevant baseline values (Table 3).
|
FF Infants (n=30) BF Infants (n=31) |
P value |
|||
Mean SD |
Mean SD |
||||
Baseline (0-14 d) |
|||||
Age, d |
7.0 |
3.1 |
5.0 |
2.8 |
0.009 |
Weight, g |
3470 |
318 |
3270 |
374 |
0.028 |
Height, cm |
49.3 |
1.8 |
48.8 |
1.7 |
0.203 |
Head Circumference, cm |
35.2 |
1.0 |
34.6 |
0.9 |
0.007 |
6 wks |
|||||
Age, d |
41.7 |
1.2 |
42.3 |
1.9 |
0.169 |
Weight, g |
4850 |
357 |
4838 |
392 |
0.249 |
Height, cm |
55.4 |
2.4 |
54 |
1.6 |
0.524 |
Head Circumference, cm |
38.1 |
1.0 |
37.7 |
1.1 |
0.559 |
12 wks |
|||||
Age, d |
83.7 |
1.3 |
84.1 |
1.5 |
0.195 |
Weight, g |
6181 |
583 |
6144 |
562 |
0.679 |
Height, cm |
59.9 |
2.5 |
59.8 |
1.5 |
0.481 |
Head Circumference, cm |
40.1 |
0.9 |
39.6 |
1.1 |
0.346 |
Table 3 Anthropometric data at baseline and at 6 and 12 wks of age
Variance analysis for repeated measures: p-values for time (stage) ´ intervention (group) were 0.266 (weight), 0.621 (height) and 0.379 (head). Methods: Greenhouse-Geisser. Covariates: gestational duration, mother’s age, delivery number, maternal education, second hand smoking, and gender.
Formula consumption
In the FF group, the mean intake of formula was 780.5±31.7 ml/d at 6weeks and 810.8±200.8ml/d at 12weeks.
Stool characteristics
Stool characteristics were evaluated at 6 and at 12weeks postnatal age based on 3days parental diaries (Table 4). The BF group had more frequent stools than the FF group at 6 and 12weeks postnatal (10 vs 4 stools/3days, p<0.05 at 6weeks and 6 vs 3 stools/3days, at 12weeks p<0.05 respectively).
|
FF infants |
BF infants |
Mann-Whitney U |
|
Median |
Median |
Z |
pvalue |
|
Number of stools and scores at 6 wks† |
||||
Stools |
4 (3, 7) |
10 (4, 15) |
-3.136 |
0.002 |
Consistency |
5.3 (4.7, 5.7) |
6.0 (6.0, 6.0) |
-4.349 |
0.000 |
Volume |
2.0 (1.3, 2.0) |
2.0 (1.3, 2.3) |
-0.887 |
0.375 |
Color |
7.3 (5.7, 8.3) |
7.7 (7.0, 8.0) |
-1.450 |
0.147 |
Number of stools and scores at 12 wks‡ |
||||
Stools |
3 (3, 4) |
6 (4, 9) |
-3.389 |
0.001 |
Consistency |
5.2 (4.0, 5.8) |
6.0 (5.5, 6.0) |
-3.398 |
0.001 |
Volume |
2.0 (1.6, 2.3) |
2.0 (1.3, 2.7) |
-0.334 |
0.739 |
Color |
7.7 (7.0, 8.8) |
7.7 (7.2, 8.0) |
-0.130 |
0.896 |
Table 4 Number of stools and scores for volume, softness, and color at 6 and 12 wks
† n=30 for the FF infants; n=31 for the BF infants.
‡ n=30 for both the FF and BF infants.
Softness Score: 1. Scattered lumps (difficult to pass); 2. Shaped like a sausage, lumps; 3. Shaped like a sausage, surface cracks; 4. Shaped like a sausage or snake with smooth surface, soft stool; 5. Soft stool, edge is clear; 6. Soft stool, the edge isn’t clear.
Volume Score (diameter): 1. <=7.5 cm; 2= 10.0 cm; 3>=13.0 cm
Color Score: 1~9 points, 1= the most unnatural; 9= the most normal
Stool consistency score was calculated based on mean of daily scores where the scores ranged from 1-6 where 1. Scattered lumps (difficult to pass); 2. Shaped like a sausage, lumps; 3. Shaped like a sausage, surface cracks; 4. Shaped like a sausage or snake with smooth surface, soft stool; 5. Soft stool, edge is clear; 6. Soft stool, the edge isn’t clear. BF infants had higher stool consistency score compared to the FF infants at 6 and 12weeks postnatal (6.0 vs. 5.2, p<0.001 and 6.0 vs. 5.0, p<0.001, respectively); however, no hard stools were observed in both groups (all demonstrated daily mean consistency score above 4, Table 4). There were no significant differences between the two groups in the scores of stool volume or stool color (Table 4).
Crying duration
The parents reported the time and duration for each crying episode during the 3days before the visits at weeks 6 and 12. The mean number of crying periods and the mean crying duration were calculated for weeks 6 and 12. Infants crying reports were limited both at 6 and 12weeks; at 6weeks, only 8(26.7%) FF infants and 5 BF infants (16.1%) reported one or more crying episodes of at least 5minutes of crying. At 12weeks, only 3(10%) FF infants and 4 (12.9%) BF infants reported one or more crying episodes, with no significant differences between the two groups. Formula fed infants had significantly higher crying duration compared to the BF infants both at 6 and 12weeks (Figure 2A). They also had more crying periods compared to the breastfed infants (Figure 2B).
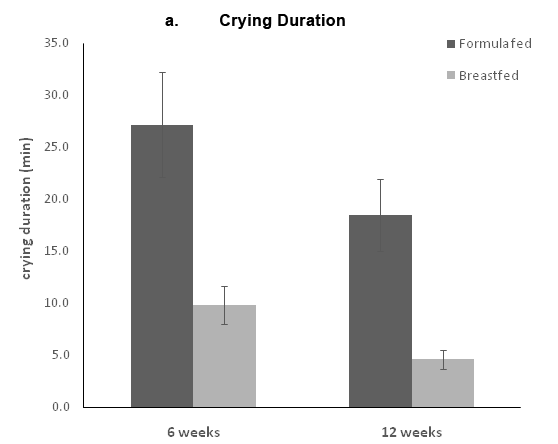
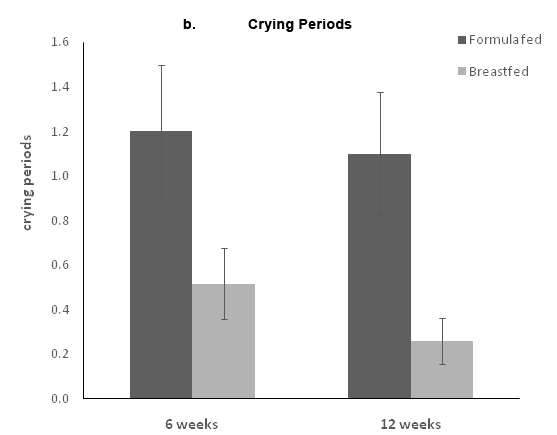
Figure 2 Crying at 6 and 12 weeks. Parents completed 3 diaries in which each crying period more than 5 minutes was recorded. The a panel represents the total crying duration, and the b panel represents the number of crying episodes. Data are presented as the means ± standard error.
Concomitant medications and adverse effects
In the FF and BF groups, 14 and 7 infants received antibiotics, 25 and 27 were given vitamins A and D and 14 and 13 were given calcium supplements, respectively. However, no significant difference in the use of concomitant medications or supplements was noted in the two groups. Twenty-four FF infants and 22 BF infants had at least one adverse effect. Of the adverse effects noted, the majority were common cold (FF 17 vs. BF 15). Other adverse effects included diarrhea, bronchitis, pneumonia, eczema, abdominal distension, and constipation. No significant difference was found in the frequency and number of specific and total adverse effect.
The present open controlled trial demonstrated, for the first time, the effect of formula containing high percentage of palmitic acid in the sn-2 position from a combination of milk fat and structured triglycerides on full term Chinese infants during the first 12weeks of life. This comparative study exhibited similarity in growth, safety, and well-being parameters between the FF and BF infants.
Few studies had compared the efficacy of high sn-2 formula with breast-milk and found a similar effect on growth in term infants. Kennedy et al.,14 found similar weight, length and HC in infants fed with high sn-2 formula compared to those fed breast milk at 12 week of age. Our study also suggests that formula with high sn-2 palmitate is an adequate substitute to breast milk in terms of infant growth. A number of studies showed that the palmitic acid in the sn-2 position improved fat absorption 8,9,11,19 and therefore may benefit infant growth.
Breastfeeding is known to be the best nutritional food for babies. Numerous studies on stool characteristics and stool frequency demonstrated that BF babies have more runny watery stools and higher stool frequency compared to FF.7,14,20 Kennedy et al.,14 compared the stool consistency among infant fed with high sn-2 formula (50% at sn-2), standard formula (12% at sn-2) and breast milk, and found that 100% of BF infants had runny or watery stools, while 14% and none in those fed with high sn-2 formula and standard formula at 6weeks respectively. Litmanovitz et al.,17 also reported less hard stools for sn-2 palmitate formula fed infants compared to regular formula. Prebiotics such as fructo-oligosaccharides (FOS) used in the study formula were suggested already to improve stool consistency21‒23 and are commonly used in infant formulas.
Although we demonstrated that infant formula containing high sn-2 and prebiotics might prevent hard or formed stools as compared with standard formula, breast milk further improves stool consistency in infants as compared with high sn-2 formula. Similar to results from previous studies,14,17,24 our breastfed infants had higher frequency of stools and softer stools than the formula fed infants. The complexity of human milk composition is not entirely understood. Human milk contains various components that together might significantly affect stool characteristics. The combination of sn-2 palmitate and oligosaccharides was also examined. Bonger et al.,24 tested the effect of beta palmitate combined with prebiotics (90% GOS, 10% FOS, 0.8gr/dl), maltodextrine and starch on stool hardness and showed a significant effect. However the Oligosaccharides levels tested in this study as well as in other representative studies,25 were over 10 fold higher than in our study. Bar-Yoseph et al reported the benefit of sn-2 palmitate on top of galacto-oligosaccharides (GOS) on fatty acids excretion mainly in the form of saponified fatty acids.10 Therefore, we assume that in our study, the main effect on stool consistency is of the sn-2-palmitate ingredient.
Though, no hard stools were reported for infants in both groups throughout the study period, differences in crying patterns were observed whereas the formula fed infants cried longer compared to the breastfed infants.
These results further strengthen study results published by Litmanovitz et al.,26 on the effect of sn-2-palmitate on crying. The effects of breastfeeding versus formula feeding on infant crying are controversial. Differences in crying between breastfed and formula-fed infants were reported. Barr et al.,26 and Lucas et al.,27 demonstrated significant differences between exclusively breastfed and formula-fed babies in crying behaviour during the first 6weeks of age. Lucas et al. revealed that breastfeeders increased their overall amount of distressed behaviour between 2 and 6weeks, whereas formula-fed infants decreased their amounts of distressed behaviour between 2 and 6weeks. An important question that was raised by Lucas et al.,27 was whether these significant feed-group related differences in infant behaviours are a consequence of differences in nutrient or non-nutrient contents of breast milk and formula.27
Moreover, studies have shown that the daily duration of crying and fussing in infants increases until 6weeks of age and then decreases progressively. Litmanovitz et al.,17 reported a peak of daily crying at 6weeks in all tested groups, with a reduction in daily crying duration at 12weeks postnatal. Our study showed a similar pattern in which the crying decreased from 6 to 12weeks. The reduction in daily crying duration was observed in all study groups and is probably a result of general maturation.
A number of limitations need to be considered in this trial. This trial was an open label and thus no randomization was performed. This can be considered a methodological flaw, as there are ethical considerations involved, especially with the infant feeding choices, which may affect the study endpoints. Our relatively small sample size limits sufficient power to detect small differences, particularly in the analyses of ordinal or categorical variables. Another limitation is that some of the data, as crying, was collected using parents diaries and questionnaires. Parent diaries of infant crying have become the standard and most widely used tool for studying infant crying; however, this method is still subjective and requires a high degree of parental cooperation.
In conclusion, although the FF infants could not reach the stool characteristics or the crying patterns as was observed in the BF infants, this tested formula, with high percentage of palmitic acid in the sn-2 position, demonstrated to maintain the wellbeing of FF infants and was found to be an appropriate alternative to breast milk for appropriate infant growth and prevention of hard stools in full-term Chinese infants in the first 3 months of life.
We would like to thank the pediatricians and nurses who were involved in recruiting the infants and in study follow-up interviews.
This study was sponsored by Enzymotec Ltd. (Israel). The An Li Cong formula was produced and provided by Wondersun Dairy, Co., Ltd, Heilongjiang Province, China. Yitong Co., China, made a financial contribution to the study. For the authors none were declared.

©2016 Zhong, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.