Journal of
eISSN: 2373-4310


Research Article Volume 2 Isuse 6
North Carolina Agricultural and Technical State University, USA
Correspondence: Salam A Ibrahim, Food Microbiology and Biotechnology Laboratory, 173 Carver Hall, Greensboro, North Carolina, 27407, Carver Hall, USA, Tel 336-285-4860, Fax 336-334-7239
Received: May 06, 2015 | Published: October 12, 2015
Citation: Obanla T, Ibrahim SA. Survival and growth of Lactobacillus Rhamnosus (ATCC 53103) in the presence of aspirin. J Nutr Health Food Eng. 2015;2(6):186-189. DOI: 10.15406/jnhfe.2015.02.00076
The objective of this study was to investigate the survival and growth of L. rhamnosus ATCC 53103 in the presence of aspirin in Lactobacilli MRS (deMan, Rogosa and Sharpe) broth. Bacterial growth was assessed by measuring turbidity, and the population was determined by pour plate method. The minimal inhibitory concentrations (MICs) of aspirin were tested by the standard agar dilution method. The effects of aspirin on β-gal activity were examined using miller units. Our results showed that significant reductions in the population of L. rhamnosus whichwere dose-dependent. The MIC was 2mg/mL with an inhibitory zone diameter of 10±0.33mm.The average β-gal activity of L. rhamnosus strain in control samples was 150±2.5Gal U. The production of β-gal decreased as the concentration of aspirin increased. Thus, our results demonstrated that aspirin can affect the growth of L. rhamnosus, and this could have an impact on human health.
Keywords: Rhamnosus, minimal inhibitory concentrations, β-gal, probiotics, gut flora, drug interaction, aging
MIC, minimal inhibitory concentrations; DMSO, dimethyl sulfoixde; FAO, food and agriculture organization; WHO, world health organization
Gut microflora play important roles in human health and disease prevention. Health benefits of gut microflora include metabolizing polysaccharides, activating the immune system, contributing towards the inactivation of pathogens in the gut, reducing the severity of rheumatoid arthritis, improving the immune response in elderly people, and regulating the host-signaling pathway.1‒3 “Probiotics are live microorganisms that, when administered in adequate amount, confer a health benefit on the host” (FAO/WHO, 2002, p. 8). Probiotics can also contribute to human health by improving the immune system and the functionality of the gastrointestinal tract against pathogenic organisms without causing any adverse effects on the host.4‒6 Probiotics have a direct impact on digestion, which also increases the nutritional value of all food products.3,7 However, the aging process may affect human gut microflora composition and functionality.1,8 The mechanisms behind the changes in gut microflora as age increases can only be speculated. For example; elderly people have reduced levels of bacteroides and bifidobacteria compared to younger people.8
In addition, bifidobacteria exhibited a reduced adhesion to the intestinal mucus of elderly people.9 Knowledge of age-related changes in the gastrointestinal tract along with changes in gut microflora could thus be an important factor in maintaining a healthy lifestyle.10 In general, the elderly have reduced immune function which could be related to the change in the composition of intestinal microflora.11,8 Changes in gut microflora could also be associated with infectious diseases in the elderly.2 In addition to aging, other factors such as diet, lifestyle, and temporary illnesses might contribute to changes in gut microflora.
Due to the increase in global life expectancy, the health status of the elderly has been gaining more attention.1 Estimates on the series of diseases among the elderly population as aging progresses have indicated that up to 85% of the elderly population is likely to be placed on one or more medications at any given time.2 Even though drug therapy could be essential when caring for patients, drug interaction with other drugs or food, or drug side-effects must be considered. Prescription and over-the-counter drugs may interact with gut microflora and modify the micro flora’s composition.12,9 For example, antibiotic drugs, which are used to kill pathogenic bacteria, will also kill other beneficial bacteria. L. rhamnosus and Bifidobacterium strains were reported to be sensitive to several antibiotics including penicillin (penicillin G, amoxicillin, piperacillin, and ticarcillin), and other anti-Gram-positive antibiotics.
Thus, it has been demonstrated that probiotics should be resistant to certain antibiotics in order to survive in the gastrointestinal tract.12,8 However; other medications may also interact with the survival or functionality of probiotics, and reduce their health benefits. Elderly patients could be at high risk of having drug interactions with probiotic bacteria. Arthritis, hypertension, and diabetic medications are among the mostly common drugs taken by elderly patients. However, the interactions between these medical drugs and probiotic bacteria have not been investigated. Previous studies have reported that aspirin and other NSAIDs could inhibit the growth of both Gram positive and negative bacteria. Thus, the objective of this study was to investigate the survival and growth of L. rhamnosus ATCC 53103 in the presence of aspirin in Lactobacilli MRS broth.
Culture activation and preparation
rhamnosus was obtained from the culture collection of the Food Microbiological and Biotechnology Laboratory at North Carolina A&T State University. The strain was activated by transferring 100μL of the stock culture into 5 mL MRS broth (Neogen, Lansing, MI) and incubated at 37°C for 18h. Bacterial cultures were streaked on MRS agar and incubated at 37°C for 48h. One isolated colony of L. rhamnosus was propagated three times in MRS broth at 37°C for 16h for the drug treatment assay.
Chemicals
All chemicals and reagents were purchased from Sigma-Aldrich Co., St. Louis, MO, USA and Thermo Fisher Scientific Co., Madison, WI, USA. Aspirin was purchased from a local store in Greensboro; NC. Acetyl salicylic acid (aspirin) was used in the experiments by dissolving in a minimum quantity of dimethyl sulfoixde (180-200μL).13
Aspirin assay
An active strain (12-16h) of L. rhamnosus strain was centrifuged at 4°C (7800×g) for 10min using Thermo Scientific Sorvall RC 6 Plus Centrifuge (Thermo Scientific Co., Asheville, NC, USA). The pellet was then washed with sterilized 0.1% peptone water (Bacto peptone, Becton Dickinson, Sparks, MD, USA) and the cells were suspended in 10mL peptone water. Aliquots of 9mL MRS broth containing different concentrations of aspirin (1mg/mL -8mg/mL) were vigorously mixed for 1min using Fisher (Thermo Fisher Scientific Co., Madison, WI, USA) digital vortex mixer (speed1500). Samples were then inoculated with 100µL of suspended cells (~7-8log CFU/mL) and incubated at 37°C. Growth was monitored every 2h for each concentration until turbidity for the control sample attained 0.9 (the stationary phase) at 610nm. Bacterial populations, β-galactosidase activity and protein expression were determined for each sample.
Bacterial enumeration
Bacterial population was determined by plating cells already exposed to different concentrations of aspirin onto MRS agar. In this procedure, exposed samples were individually diluted with a serial of 9mL sterile 0.1% peptone water after which 100μL of appropriate dilutions were surface plated onto triplicate MRS agar and incubated at 37°C for 48h. Plates with colonies ranging between 25 and 250 were enumerated to determine the bacterial populations.
β-Galactosidase activity assay
β-Galactosidase activity was determined following the method described by Ibrahim and O’sullivan14 with some minor modifications. Bacterial growth was determined by measuring optical density at 610nm using Thermo Scientific Genesys 10S UVV is spectrophotometer (Thermo Fisher Scientific Co., Madison, WI, USA). An aliquot of 100µL of each sample was added to 900µL each of Z buffer (0.06M Na2HPO4; 0.04M NaH2PO4; 0.01M KCL; 0.001 M MgSO4.7H20) to obtain a final volume of 1mL. Ten microliters (10µL) of chloroform were added to each sample and then shaken for 30min in the incubator at 37°C. Reactions were triggered by adding 200μL of ο-nitrophenyl-β-D-galactopyranoside substrate (4mg/mL in 0.1M phosphate buffer) to the samples and reactions were allowed to proceed at 37°C until a strong yellow color was developed. The time for the development of a strong yellow color was recorded; the reaction was then stopped by adding 5µl of 1M Na2CO3 and optical density was measured at 420nm and 550nm for each sample. Units of β–galactosidase (β-gal) were recorded as miller units using the following equation.
100, [OD(420 nm)−175 × OD(550 nm)/(T × V × OD(600 nm)]
where T is the time and V is the volume.
Determination of minimum inhibitory concentration (MIC)
A diffusion assay was conducted to determine the MIC unit of aspirin. Aliquots of MRS agar with 0.2% Tween 80 were prepared and sterilized at 121°C for 15min. The agar was placed in water bath at 49°C and allowed to cool. Agar was inoculated with L. rhamnosus ~7-8 log CFU/mL to achieve an inoculums level of ~7-8 log CFU/mL and then poured into Petri dishes (100x15mm), allowed to solidify, and the agar plates were kept at 4°C overnight. One well (~8mm in diameter) was made at the center of the MRS agar plate using a sterile test tube. Each well was filled with a different concentration of aspirin dissolved in dimethyl sulfoixde (DMSO). Plates were incubated for 48h at 37°C and then examined for clear inhibition zones around the well every 3h. The assay was carried out in duplicate for all concentrations of aspirin. The size of the zone of inhibition was calculated as the diameter of the zone minus the diameter of the bored test well. The MIC was defined as the lowest concentration that inhibited growth of L. rhamnosus. Independent experiments were repeated three times and the average inhibitory zone was recorded.
Statistical analysis
Different concentrations of aspirin were statistically analyzed for their impact on growth and functionality of L. rhamnosus by factorial analysis of variance of duplicate samples. Each experiment had three replicates to determine statistical differences. Data from all three experiments were pooled to calculate the statistical mean and standard error of the mean for each treatment. Data were verified for normality using IBM SPSS 2009, followed by two-way analysis of variance (p<0.05) using SAS version 9.2 (SAS Institute, Cary, NC).
Figure 1 shows the growth of the L. rhamnosus strain after incubation at 37°C for 9h. The bacterial strains continued to grow during the incubation period, and turbidity readings reached ~0.90 after 9h in the control samples. With the addition of 1mg/ml of aspirin, the growth showed no significant difference from the control sample. However, with higher concentrations of aspirin, there were noticeable changes in growth as indicated by lower turbidity compared to the control. The optical density of the sample decreased as the concentration of aspirin increased (P≤ 0.05). The result showed that during the first 5h of incubation with higher concentrations of aspirin of 5-8mg/mL, growth was completely inhibited. At the end of the incubation period, the population of all aspirin concentrations was determined (Figure 2) (Figure 3). In control samples, the population of L. rhamnosus increased from ~3log CFU/ml to ~8.8 log CFU/mL after 9h. The population count for 1mg/mL of aspirin showed a similar result with the control (P>0.05); however, the addition of 2mg/mL of aspirin resulted in approximately one log cfu/mL reduction in population. Moreover, the result indicated that the mortality of L. rhamnosus increased with higher concentrations of aspirin. Cell viability after exposure to aspirin revealed a concentration-dependent reduction (p<0.01). The β-galactosidase activity of L. Rhamnosus in response to different concentrations of aspirin is shown in Table 1. In the control sample, L. Rhamnosus produced a significantly higher β-galactosidase activity of approximately150±2.5 (p<0.01) compared to the treated samples. Lower concentrations of aspirin significantly decreased the production of β-gal in L. rhamnosus which indicated an inhibition of activity as shown in Table 1. Similarly, the β-galactosidase in L. rhamnosus was found to be completely inhibited by aspirin concentration ≥ 3mg/mL. The activity results showed a concentration-dependent inhibition (p<0.01).
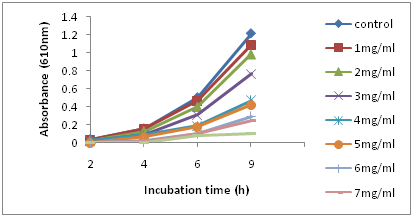
Figure 1 Survival and growth of L. rhamnosus in MRS broth in the presence of aspirin at different concentrations during incubation at 370C for 9h.
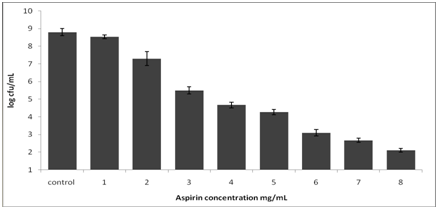
Figure 2 Population of L. rhamnosus in MRS broth with different concentrations of aspirin after incubation at 370C for 9h.
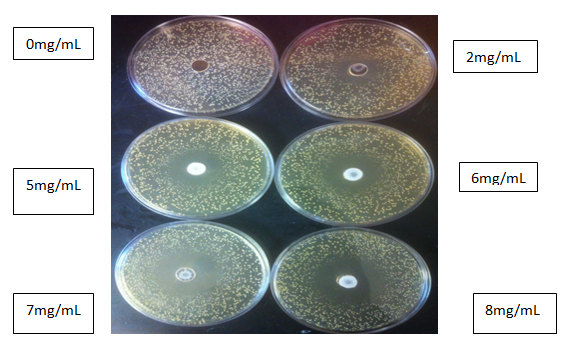
Figure 3 Inhibitory effects of different concentration of aspirin (mg/mL) after 48h of incubation at 37°C.
Treatment = different concentration of aspirin (mg/ml).
Aspirin |
β-Gal Activity |
Control |
150± 2.5 |
1 |
20.0 |
2 |
10.0 |
3 |
0.00 |
4 |
0.00 |
5 |
0.00 |
6 |
0.00 |
7 |
0.00 |
8 |
0.00 |
Table 1 Aspirin concentration on β-Gal activity
*Assays were done using permeabilized whole cells, and units of activity were calculated as described by Miller (1972).
N=3
Table 2 shows the value for minimum inhibition concentrations of aspirin. As the concentration of aspirin increases, the MIC value increases (p<0.05). Based on this result, the MIC was 2mg/mL with an average zone diameter of 10±0.33mm. The inhibitory zone diameter increased to 13±0.33 when 3mg/mL were added. The highest zone of inhibition of 19±0.05mm was observed with a concentration of 8mg/mL. This finding suggests that aspirin has antibacterial effects on L. rhamnosus and is also dosage dependent.
Aspirin |
MIC Observed diameter of zone (mm)* |
Control |
No Inhibition |
1 |
No Inhibition |
2 |
10±0.33 |
3 |
13±0.33 |
4 |
15±0.21 |
5 |
15±0.11 |
6 |
15±0.11 |
7 |
16±0.07 |
8 |
20±0.05 |
Table 2 Minimum inhibitory concentration (MIC)
*Average of three replicates
MIC: Minimum Inhibitory Concentration
Zone of inhibition = (Diameter of the zone – (diameter of the bored test well).
Gut microflora plays important roles in human health and disease prevention. Poor food choices, emotional stress, antibiotics overuse, and environmental factors can shift the balance in favor of harmful microorganisms. Administration of medical drugs has also become a daily routine among the elderly population. These medications are used to treat disease and to improve health. It is important to understand that all medicines, both prescription and over the counter, have risks as well as benefits. Intake of medical drugs could negatively alter the composition of natural flora in the gut and reduce the population of beneficial bacteria. This alteration would promote proliferation of pathogenic organisms in the gut resulting in diarrhea, urinary tract infections, muscle pain and fatigue. The change could also lead to a reduction in the functionality of gut flora such as metabolizing polysaccharides, activation of the immune system, and improving the immune response in the elderly. Healthy individuals need to maintain normal gut flora which confer health benefits. Our study suggests that the consumption of probiotics is critical to the maintenance of this healthy level of human gut flora. Probiotics could thus help maintain and contribute to human health by improving the immune systems and the functionality of the gastrointestinal tract against pathogenic organisms without any adverse effects on the host.
In this study, we examined the survival and growth of L. rhamnosus in the presence of different concentrations of aspirin, and we found that aspirin significantly reduced the growth of L. rhamnosus and β-gal activity. The fact that aspirin could alter the growth of L. rhamnosus was reported in our previous study,15,16 which also indicated that common medicines such as aspirin could shift the growth of probiotics including Bifidobacterium longum (ATCC15707) and Lactobacillus reuteri (ATCC 53608). Although the mechanism by which aspirin inhibits the growth and β-gal activity of L. rhamnosus was not investigated in this study, it would appear that aspirin can inhibit protein synthesis and thereby affect the behavior of L. rhamnosus. As a result, additional work is needed to investigate the mechanism that impacts the survival and growth of natural flora including L. rhamnosus in the presence of aspirin. The results of this additional research could positively impact the health and quality of life of an increasingly large and aging population.
This work was supported by the Agricultural Research Program at North Carolina Agricultural and Technical State University with funding from the USDA National Institute of Food and Agriculture, Hatch project number NC.X-234-5-09-170-1. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Food and Agriculture.
Author declares that there is no conflict of interest.

©2015 Obanla, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.