Journal of
eISSN: 2373-4310


Research Article Volume 13 Issue 3
1Abode Biotec, India
2CSIR-Central Salt and Marine Chemicals Research Institute, India
Correspondence: Mayuri Banerjee Bhattacharya, Abode Biotec India Pvt. Ltd., Hyderabad, Telangana, India
Co-correspondence: Mr. Ranjith Kumar Kallur, Abode Biotec Research and Techno Commercial Department, Chief Technical Director, Abode Biotec, India, Tel +91-9885479749, Tel +91-7043431408
Received: July 02, 2024 | Published: July 22, 2024
Citation: Kallur RK, Madapati S, Mathur A, et al. Study the efficacy of ProBC plus (bacillus coagulans LMG S-31876) among the participants through evaluating protein absorption & GI symptoms: a prospective, randomize control clinical trial. J Nutr Health Food Eng. 2024;13(3):14-19. DOI: 10.15406/jnhfe.2024.13.00377
Background: Globally, the probiotics are often defined as live microorganisms that provide various health benefits. One such probiotic strain is Bacillus Coagulans, which has emerged as a promising strain for its role in the enhancing protein absorption that is a critical component of overall nutritional health.
Methodology: The current study is a randomized control clinical trial to test the efficacy of probiotic ProBC Plus (Bacillus Coagulans LMG S-31876) product for 8 weeks. This double-blind, randomized control study, the two parallel groups having 14 individuals in test group and 16 individuals in the placebo group were monitored closely. Furthermore, major GI symptoms as well as protein absorption over 8 weeks was observed.
Results: ProBC Plus (Bacillus Coagulans LMG S-31876) did not aggravate or have any adverse effects on GI disorders/symptoms during the course of consumptions and outcome was statistically significant. Furthermore, the study shows a average urine urea nitrogen among the test group was statistically significant when compare with placebo group and within the normal reference range. Thus, ProBC Plus (Bacillus Coagulans LMG S-31876) has the potential to optimize protein utilization efficiently.
Conclusion: Research reveals that ProBC Plus (Bacillus Coagulans LMG S-31876) not only enhances protein absorption but also promotes better overall digestive health.
Keywords: probiotics, ProBC Plus (bacillus coagulans LMG S-31876), randomised control trial, efficacy trial, clinical study, gi symptoms, protein absorption
Globally the probiotics have been classified by the Food and Agriculture Organisation (FAO) and the World Health Organization (WHO) as "live micro-organisms," which, when given in sufficient proportions, boost the host's health (FAO/WHO, 2006). One of the biggest markets for functional foods (FF) is probiotics, which are based on the utilization of microorganisms to maintain or restore health. These have gained an increasing attention for their potential to influence various aspects of human health.1 One such strain is Bacillus Coagulans has emerged as a promising candidate for its role in the enhancement of protein absorption that is a critical component of overall nutritional health.
The probiotics are thought to have a wide range of positive effects on health in today’s scenario through regulating the growth of different good bacterial species in the stomach, supporting the integrity of the intestinal barrier, and enhancing certain aspects of the human immune system.2 In addition, probiotics have the ability to reduce the ability of pathogens to adhere to host tissue and regulate the synthesis of several metabolites, including vitamins, short-chain fatty acids, and compounds that function as neurotransmitters in the gut-brain pathway.3 Probiotics have been demonstrated to affect the absorption and production of important nutrients, such as minerals, carbohydrates, protein, cholesterol, and other types of digestive enzymes, in addition to their physiological benefits.4–6 Protein absorption is a fundamental physiological process that plays a pivotal role in the maintaining the tissue integrity, facilitating of metabolic functions, and supporting muscle growth within the body.7 There are several factors that influence the efficiency of protein uptake in the gastrointestinal tract such as age; micro-biota composition, and diet, health conditions can impact the efficiency of uptake of protein in Gastro intestinal tract. Thereby, protein absorption has garnered significant interest.8,9 B. coagulans produces enzymes that have been shown to aid the breakdown of protein and a wide variety of carbohydrates.10 In addition to the potential role in the protein absorption, probiotics have been studied to enhance the absorption process of small peptides and amino acids by elevating the absorptive capacity of the intestinal epithelium and thereby, facilitating transport mechanisms. The ability to improve the gut lining and inflammation11,12 and the increase in the production of digestive enzymes are the candidate mechanistic links for Bacillus Coagulans to improve the absorption of amino acids into the bloodstream as well as improve protein digestion from both milk and plant proteins.13,14 Various sources of proteins are available for human diet, however, each of this protein sources exhibit divergent digestive kinetics. Due to these differences, the appearance of amino acids is impacted as well as their ability to stimulate changes in protein metabolism.15,16 The utilization of probiotics can also aid in reduction of toxic metabolites production in the gut.13
Despite of these intriguing results and findings, there is a necessity for a well-designed clinical trials to assess the effects of Bacillus Coagulans based product on protein absorption in humans. This is required due to the complexity of the gastrointestinal environment.17 Bacillus Coagulans LMG S-31876 is a well-characterized probiotic strain belonging to the Bacillus species and is notable for its spore-forming nature. It was initially isolated from fermented rice samples and has since undergone extensive research and characterization.
This particular strain of Bacillus coagulans, LMG S-31876, is securely deposited and referenced as "ProBC Plus." It has been officially cataloged and can be accessed through the Belgian Coordinated Collections of Microorganisms/LaboratoriumvoorMicrobiologie (BCCM/LMG) under the accession code LMG S-31876. Additionally, it is available for research purposes through the Microbial Type Culture Collection-International Depositary Authority (MTCC-IDA) under the Budapest Treaty, with the accession number MTCC 25396. Further accessibility is provided by the American Type Culture Collection (ATCC), where it is identified with the designation ATCC SD-7789.18
The aim of the present work is to study the overall health effects of ProBC plus (Bacillus Coagulans LMG S-31876) on the participants for the duration of 8 weeks trials, through advance randomized control efficacy trial. The objectives are: (a) to examine the efficacy of ProBC Plus (Bacillus Coagulans LMG S-31876) protein absorption on the participants for 8 weeks trial, where main indicator is urine urea nitrogen level. (b) to assess the GI symptoms of the test group and placebo group during for 8 weeks (c) to monitor the vital health parameters as well as hematological values of the participants consuming ProBC Plus (Bacillus Coagulans LMG S-31876) during the trials periods of 8 weeks.
Study design and selection of participants
This is a pilot scale; randomize control trial (RCT) to evaluate the efficacy of the ProBC Plus (Bacillus Coagulans LMG S-31876) on the test and placebo groups. The main purpose of the study was to evaluate the efficacy of the ProBC Plus (Bacillus Coagulans LMG S-31876) on the subjects relating of major macro nutrients absorption for duration of 8 weeks. In the current research is a double-blinded randomize control efficacy trial, Declaration of Helsinki as well as local and legal bodies regulations and guidelines were strictly followed. Moreover, subject information sheets (SIS) were provided to all the study participants and informed written consent was obtained prior to the test trials.
To closely monitor the study subjects, the sample size was kept 30, with age group between 18 to 50 years respectively; furthermore participants were divided into two control groups. However, in the process of random allocation of the participants, 14 participants were in test group and 16 were in placebo group (Figure1).
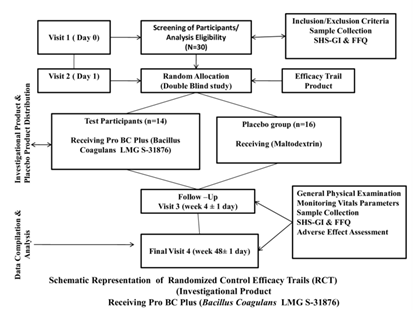
Figure 1 Schematic representation of Randomize Control Trial – Investigate the efficacy of ProBC Plus (Bacillus Coagulans LMG S-31876).
The demographic data such as age, gender, past and present medical history (co-morbidity), history of allergies, life style and dietary pattern were noted. The study participants were included only when all the all information’s were obtained was true to their knowledge and they are willing to participate in the research study by giving written consents.
Additionally, subjects having oral abusive habits like consumption of tobacco as well as those who consume alcohol were excluded from the RCT. At the same time, pregnant women and lactating mothers, person with food and drug allergies, as well as the participants who have undergone any surgery was excluded from the study. The ethical clearance was obtained from all the concern authorizes, and at the same time, the main focus was the retention of the participants for 8 weeks and minimized the dropout rates among the participants.
Study material and data collection
To collect the baseline data, both close and open ended questionnaires where designed. Additionally, the questionnaires were tested and validated as well as modifications were done as per the local setting. The questionnaires were translated both in national as well as regional languages for better understandings. Short Health Scale for GI symptoms (SHS-GI) and food frequency questionnaires (FFQ) were administered during the periodically and at regular interval during visit 1 to visit 4. Besides this, the clinical parameters such as monitoring the vital signs of the study participants, collection of blood and urine samples were done during subsequent visits.
Investigating procedure, product information and placebo
The entire study was divided four major visits. In the first visit (Day 0) baseline screening, selection of health individual, obtaining the written consent was and questionnaires were administered. At the same time all vitals (Systolic, Diastolic, Weight, Height and Plus rate) were collected. Furthermore, blood and urine samples were also collected and analyzed.
On the second visit (Day 1) 30 study participants were allocated randomly in two groups with product test group having 14 individuals and placebo group with 16 individual. Further, during the same visit along with general physical examination of the participants, all the vital signs were monitored, and product samples were given. Through instructions were given to the study participants regarding consumption. On the visit 3 (week 4 ± 1 day) and visit 4 (8 ± 1 day), general physical examination of the participants, all the vital signs were monitored, blood and urine sample collections. The assessment of SHS-GI and FFQ was done along with investigational products compliance and reconciliation was reported.
Investigational product (IP)
Each 1g ProBC Plus (Bacillus Coagulans LMG S-31876) sachet contains NLT 1 billion CFU of Bacillus Coagulans LMG S-31876, which appears to be an off-white color powder, filled in small pouches and sealed properly. Thus, the test participants were provided with small sachets of ProBC Plus (Bacillus Coagulans LMG S-31876) for daily consumption. The ProBC Plus (Bacillus Coagulans LMG S-31876) sachet was stored in cool and dry place. Besides this, 14 test participants were given the test product sachets and periodic refill of the test product was done to maintain the continuity. On the other hand, the placebo groups were given similar colored small sealed sachets, weight 1 gm containing (Maltodextrin) starch for the regular consumptions during the study periods.
Treatment regime and period
In the current study 14 were in the test group and 16 were in the placebo group, however, neither the investigator nor the study subjects were aware of the test line and placebo side. Among 30 participants, 14 test subjects were given the test product sachets and periodic refill of the test product was done to maintain the continuity. Likewise, among 30 participants, 16 individuals in placebo groups were give the identical starch sachets. The sachets of active product and placebo has to be taken orally, consume 2 sachets with water daily at approximately same time each daily (preferably morning and night with glass of water) and time of intake should be approximately should be same for each day of the study period.
Data interpretation and statistical analysis
Post data collection, the analysis was conducted as per the statistical analysis plan (SAP) and the SAS software version 9.1 and graph pad. The mean and standard deviation along the p value is reported in the result. Further, the difference within and between the two groups was assessed by using student t-test to test the significance. Graphs and figures are illustrated in the result and discussion.
Efficacy and safety variables
The changes within the group and intergroup were identified from the mean change from baseline in urine urea nitrogen assessment, SHS-GI and Food Frequency Questionnaire (FFQs) at baseline (visit 1), visit 3 (week 4) and visit 4 (end of study at week 8) for both the groups. The primary and secondary outcomes include the evaluation of the effect of Bacillus Coagulans on protein absorption at Baseline, Week 4 and Week 8 and analyzing the safety of the study product during intended use.
In this current randomized control efficacy trial, 14 individuals (5 females and 9 males) wherein test trial group received ProBC Plus (Bacillus Coagulans LMG S-31876) daily divided doses, whereas, 16 individuals (6 females and 10 males) wherein placebo groups. The overall mean age of the participants was in test trial group is 33.71 ± 9.95 years and placebo group is 33.94 ± 7.61 years. Thus mean age clearly shows that the study participants belong to young age group. The mean weight of 30 individuals both test group and placebo group is 60.7 ±7.5 Kg and mean height of the participants is 163.5±10 Cm, thus average BMI (BMI = kg/m2) of the study participants was 22.8 ± 2.2 kg/m2. Hence, the individuals who participated the RCT efficacy trial, was in good state of health and had better absorptions of the product.
Assessment of vital parameters among the study participants
Vitals parameters such as pulse rate, oral temperature, systolic and diastolic blood pressure were found to be in normal range during the course of the study. Furthermore, when the vital signs of test group consuming the test product ProBC Plus (Bacillus Coagulans LMG S-31876) as well as placebo group was remained static and identical for 8 weeks of the trial period. Hence, it is concluded that there was no significant change in the vital parameters both in test and placebo group. Similarly, the vitals exhibited a normal range. The statistically significant differences observed with respect to baseline values indicate that neither ProBC Plus nor the placebo had a detrimental effect on these vital signs during the study period (Figure 2).
Investigation of the food frequency questionnaire (FFQ) to assess the dietary intake (energy, proteins and fats, carbohydrates) among the participants
The figure illustrates (Figure 3) the dietary pattern of the participants through the Food Frequency Questionnaire (FFQ), in which the energy intake (in Kcal), protein, fats and carbohydrates (Figure 4 (a-d) between the test group and placebo groups for 8 weeks duration was assessed. The average per day calorie consumption among the participants was found to be 3680±1588 Kcal. Moreover, it was revealed during the course of study that there was slight decrease in the average calorie consumption in both the groups. Although, these results directly do not influence protein absorption, the alterations in energy intake could have implications for overall nutritional intake and thereby indirectly impacted protein absorption.
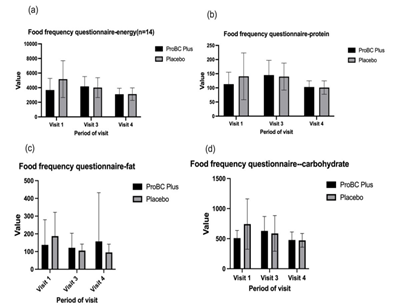
Figure 3 Investigation of the Food Frequency Questionnaire (FFQ) to assess the dietary intake among the test group (ProBC Plus) and placebo group.
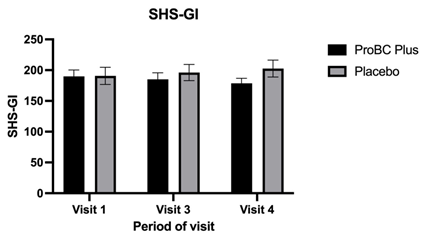
Figure 4 Evaluation of SHS-GI (Short Health Scale) for gastrointestinal symptoms among the test group (ProBC Plus) and placebo group.
Additionally, the major macronutrients such as carbohydrate, fat and protein, fat consumption pattern was also assessed for 8 weeks among all the individuals. However, there the data shows no significant changes related to the intake of the major macronutrients.
Although, FFQ did not reveal statistical significance difference in energy, fat and protein, carbohydrates between ProBC Plus and placebo the change in dietary can impact nutrient absorption and health. The relationship between dietary patterns and nutrient absorption plays an important parameter to analyze the bioavailability of the nutrients.
Evaluation of SHS-GI (short health scale) for gastrointestinal symptoms
In the present study, GI symptoms such as change in bowel movement (constipation and diarrhea), nausea, vomiting, decrease or increase in appetite, acid reflux, abdominal pain (epigastric, upper and lower abdomen as well as pelvic region) among study participants was recorded. It was found that, from visit 1 (day 0) till visit 3 (4 week ± 1 day) for the test group, there was slight decrease in the GI symptoms. However, for the same visit for the placebo group GI symptoms gradually increased. Additionally, on the final visit (8week ± 1day) for the test trial group consuming ProBC Plus (Bacillus Coagulans LMG S-31876) daily divided dosage the GI symptoms were significantly reduced (p<0.0001). On the other hand the placebo group the GI symptoms gradually changed and some of the major GI symptoms were reported among the placebo groups (Figure 4). The GI symptoms can affect individual’s ability to digest nutrients and absorbs proteins, ProBC Plus effectively reduces GI symptoms, and it may indirectly contribute to the protein absorption. When GI tract is functioning smoothly with reduced discomfort, the ability of the break down and absorption of the proteins is a positive indicator. Thereby, enhanced protein absorption is a positive indicator good health and wellbeing among individuals.
Assessment of hematology parameters
The study shows the blood serum parameters between the test group and the placebo group. The research shows that the mean hemoglobin among the 30 study participants during the initial visit (Visit 1) was 13.9 ± 1.6 gm/dl which was gradually increased to 14.2 ± 1.3 gm/dl among the 14 test group indicated in (Table S1) depicted a normal level between placebo and were within the range and there was a statistical significance between placebo and ProBC Plus. The other parameters for hematology have been depicted in supplementary information (Figure 5). Similarly, depicted the stability of vital parameters within the normal range and thereby suggested that probiotics should not have adverse impact on the safety profile.
Analysis of urine urea nitrogen for protein absorption
In the clinical tail, the efficacy ProBC Plus majorly depends on the urine urea nitrogen absorption on the test group and placebo. The average urine urea nitrogen among 30 participants was 275.5 ± 182.2 mmol/day during visit 1 (Day 0) which was less than the reference range (428.4 to 714 mmol/day by UCSF Health, 2021),19 analyzed prior to the randomization and initiation of the efficacy trial. As the urine urea nitrogen is one of the key indicators of protein metabolism, hence in the study it was closely monitored for 8 weeks duration post randomization and double blinded allocation of participants into the group. During the visit 3 and visit 4, the average urine urea nitrogen among the test group was 348±78 mmol/day and 366±147 mmol/day respectively. In contrast to this, the average urine urea nitrogen among the placebo group during visit 3 and visit 4 was 202.1±85.7 mmol/day and 164±85.7 mmol/day as illustrated in the (Figure 6). Moreover, on comparison of the average urine urea nitrogen between the test and placebo group the data reveals that ProBC Plus do not have any undesirable effect on the protein metabolism for 8 weeks as well as the urine urea nitrogen level was also increased within the normal range. The urine urea nitrogen level during the end 8 weeks of the study was statistically significant (P<0.0001) when compare within the test and placebo group. ProBC Plus exhibited a positive effect on urine urea nitrogen as compared to the placebo. Similarly, ProBC Plus and placebo, there is a statistical significance (P<0.0001) at baseline and week 8 predicting the ProBC Plus a positive impact on urine urea nitrogen as compared to the placebo. The observed results have been associated with enhanced protein utilization due to the potential to modulate gut microbiota that promotes the environment that is efficient for nutrient digestion and absorption.20,21 Thereby, ProBC Plus exhibited an improvement in protein absorption.
In the present randomize control clinical trial, the efficacy of ProBC Plus (Bacillus Coagulans LMG S-31876) was assessed through SHS-GI (Short Health Scale) for monitoring GI symptoms and Food Frequency Questionnaire (FFQ) to evaluate the dietary pattern of study subjects were use. Along with the questionnaires, blood and urine samples were periodically collected. As study was divided into 4 visits for duration of 8 weeks between two groups (test group n=14 and placebo group n=16), it is concluded that there was gradual decline in the GI symptoms during the course of consumption of ProBC Plus (Bacillus Coagulans LMG S-31876). The reduction in GI symptoms among test group was found to be statistically significant. Additionally, urine urea nitrogen absorption of ProBC Plus (Bacillus Coagulans LMG S-31876) among the test group was statistically significant when compared with the placebo group by the end of visit 4. Thus it is concluded that ProBC Plus (Bacillus Coagulans LMG S-31876) is potentially efficient and affirmative effects on GI symptoms and protein absorption.
Even though, the results provide valuable insights, further mechanistic approach is required and this study serves as a base for further research in the dynamic field of probiotic products. Nonetheless, the study underscores the multifaceted relationship between dietary habits, gut health and nutrient absorption thereby, emphasizing the potential of ProBC Plus to contribute to improved overall health and nutrition.
We would like to thank Bio Agile Therapeutics Pvt. Ltd, Bengaluru for the clinical research services, and Medstar Specialty Hospital, Bengaluru for the conduction of the trials. The authors would like to thank the patients who participated in this trial.
RK: Conceptualization, Formal Analysis, Project Administration. SM: Project Administration, Resources, Supervision, Fund Acquisition. AM: Data Interpretation, Formal Analysis, Investigation, Methodology, Validation, Writing- Reviewing, Software. SB: Writing – review & editing. MBB- Rephrasing Methodology, Data Interpretation and Final Draft Preparation.
The study is registered in CTRI (Clinical Trial Registry India) with CTRInumber CTRI/2023/04/051660.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Medstar Specialty Hospital, Bengaluru. The patients/participants provided their written informed consent to participate in this study.
The authors declare no competing interests.
This research was funded by Abode Biotec Private limited.

©2024 Kallur, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.