Journal of
eISSN: 2373-4310


Research Article Volume 7 Issue 3
1Department of Biological Sciences, Salisbury University, USA
2Food Science and Technology, Department of Agriculture, University of Maryland Eastern Shore, USA
Correspondence: Kumudini A Munasinghe, Department of Biological Science, Salisbury University, Salisbury, USA, Tel +1 410-548-4242, Fax +1 410-543-6433
Received: August 22, 2017 | Published: November 14, 2017
Citation: Munasinghe KA, Schwarz JG. Rooster collagen extracts from rooster by-products. J Nutr Health Food Eng. 2017;7(3):296-299. DOI: 10.15406/jnhfe.2017.07.00239
Collagen extracts are useful for industrial applications due to their high-water retention capacity, ability to repair rough skin, lack of color and odor problems, absence of harmful effects on human health, and low incidence of causing allergic reactions. The extraction of collagen from rooster skin could lead to an alternate source of collagen for various industries including the food industry. The collagen yields and their physical and chemical properties can be varied not only with the source but also with the treatment methods. The objective of this research was to extract collagen from rooster skin by utilizing traditional methods and pepsin digestions to compare the yields of different collagen extraction methods. 2.6M NaCl was used to precipitate collagen from pretreated skin in the presence of 0.05M Tris (hydroxymethyl) aminomethane (pH 7.0). Statistical analyses were performed using CRD and the Student’s t-test, with a P < 0.05. The average yield obtained from traditional methods was 6% in dry weight basis, while the average yield obtained for pepsin digestions was approximately 37%. Molecular weights of collagen extracts from acetic acid, citric acid, alkali extractions were over 100kDa while those from pepsin extractions were below 66kDa because of the further digestion of collagen in pepsin. Acid solubilized rooster collagen had similar banding patterns as acid solubilized calf skin collagen. This indicates that the rooster collagen can be considered as an alternate source of collagen for many applications.
Keywords: rooster collagen, collagen extractions, collagen yields
Collagen associated hydrolysates have desirable characteristics for various industrial applications.1,2 They have been used as important biomaterial in medical applications because of their biodegradability and weak antigenicity.3 In addition, intra-cellular and extra-cellular self-assembly of polypeptide chains and the cross-links between adjacent molecules help collagen fibrils to withstand physical stress.4 Collagen is also effective in decreasing cooking loss of deli products containing chunked chicken meat by increasing protein-protein bindings, and has the potential to improve the quality characteristics of deli rolls by improving their texture.5 Further, Mittal6 showed that collagen hydrolysates can be used as binders or extenders in meat emulsions. Moreover, collagen can also be used to make natural casings for sausages and frankfurters7 and to fill periodontal bony pockets.8 Collagen is however, deficient in the essential amino acid tryptophan and limited in some essential amino acids such as lysine, threonine and methionine.9
Collagen has been used for cosmetic and medicinal purposes since 1975, including for the reduction of wrinkles.10 Millions of patients have been treated with collagen and approximately 280 clinical studies have been published.10 This natural substance present in the connective tissues is used for the reconstitution of the dermis and for dermal wound healing.11 It has been shown that skin replacement with a collagen-based dermal substitute gave good functional and cosmetic results.12
Collagen fibers can be seen at the time that structure differentiation begins in early embryonic development.4,13 Diegelmann14 reported that adult skin contains about 80% type I and 20% type III collagen. In newborns, the type III content is greater than that of the adults. Collagen is one of the most abundant proteins on earth.15 The occurrence of collagen in invertebrate species was studied by Eastoe & Pikkarainen et al.,16,17 who found large amounts of collagen in octopuses, earthworms, fish, tapeworms and sea mussels.
The denaturation temperature of mammalian collagen is higher than that of collagen obtained from marine animals. Further, the mammalian collagen denaturation temperature is compatible with human body temperature, 37°C, and is therefore extensively used for medical purposes.3 Chicken collagen can be utilized as an alternate source for biomedical purposes because denaturation temperature for chicken collagen was estimated to be 38°C by Sakai et al.18
Morimura et al.,19 used pig skin to extract collagen using acetic acid and alkali (NaOH, pH 12) solutions. In the same study, fat and inorganic materials were removed prior to collagen extraction and collagen yields for defatted pig skin with acetic acid and alkali extractions were 72% and 80%, respectively. Another study reported that collagen yield extracted from the cuttlefish skin with acetic acid was 2% and that of pepsin digestion was 35%.20 It was also shown that efficiency in collagen extraction from the skin of bigeye snapper could be enhanced by incorporating pepsin at 20kU/g using defatted skin during 48h extraction, after an acid pre-swelling treatment for 24hours.21 This study obtained 54% collagen yield after 48hours for bigeye snapper skin. Dry weights of chicken collagen extractions for acetic acid, citric acid, sodium hydroxide, one-step acetic acid and one-step pepsin, and two-step acetic acid and one-step pepsin for the skin extractions were 6.1, 6.2, 5.0, 38.7, and 40.4 percent, respectively and those of bone extractions were 4.4, 4.1, 4.1, 19.1, 20.6 percent, respectively.22 Base on the yields obtain from chicken collagen in Munasinghe et al.,22 higher yields can be expected from rooster skin as an alternate source of collagen with the same five different extraction methods than from rooster bones. Further, the rooster skin collagen can be expected to have better mechanical properties than broiler chicken collagen, because mechanical properties of collagen varies with the fibril size, orientation, age, and anatomical location23 of the source. One-and a half year old rooster might have collagen that has better mechanical properties than the collagen extracted from 45days old broiler chicken. In addition, the yield obtained for different extraction methods can vary with the treatments utilized. The objective of this study was to extract and analyze rooster collagen, and compare yields obtained for different collagen extraction methods.
Rooster skin preparation
Rooster skin was obtained from a commercial vender (Perdue chicken Salisbury, MD) and stored at 4°C until used. The skin was washed, and pretreated to remove non- collageneous protein, fat, and inorganic compounds with 0.1N NaOH, 10% butyl alcohol, and 0.1N HCL, respectively.22,24 Two replicates were used for each extraction method, and all procedures were conducted at 4°C with continuous stirring. The NaOH solution was changed every 2hours to remove non-collagenous proteins over 6hours, and the samples were washed with distilled water. Deproteinated samples were stirred in 10% butyl alcohol with a solid/solvent ratio of 1:6 (w/v) for 48hours to remove the fat. The butyl alcohol was changed every 2hours, and samples were washed with distilled water prior to the removal of inorganic compounds. The defatted samples were stirred in a 0.1N HCl solution for 24hours with a solid/solvent ratio of 1: 6 (w/v) to remove inorganic compounds. The pretreated samples were washed three times in distilled water prior to conducting collagen extraction procedures.
However, it was very difficult to conduct pretreatment steps with rooster skin because of the thick fat layers attached to the skin. Therefore, fat removal time for the experiment was increased up to 48hours with 10% butyl alcohol. The longer processing time, along with difficulties in continuous stirring, and filtering were disadvantages of utilizing rooster skin for collagen extractions.
Collagen extraction: Collagen extractions were conducted using five different extraction methods; acetic acid, citric acid, alkali, one-step acetic acid and one-step pepsin, and two-step acetic acid and one-step pepsin extractions.
Acetic acid extraction: 0.5M acetic acid with a solid/solvent ratio of 1:6 (w/v) was used to extract collagen from rooster skin. After 48hours of collagen extraction, the sample was filtered using cheese cloth, and filtrate was used for collagen precipitation.24
Citric acid extraction: 0.5M citric acid solution with a solid/solvent ratio of 1:6 (w/v) was used to extract collagen from rooster skin for 48hours. The dissolved collagen in citric acid was purified by dialyzing it in distilled water at 4°C and the filtrate was used for the precipitation.18
NaOH extraction: minced rooster skin was stirred in NaOH (pH 12) with a sample solution ratio of 1:6 (w/v) at 4°C for 48hours. After extraction, the sample was filtered through double layer cheese cloth, and the filtrate was used for precipitation.19
One-step acid and one-step pepsin extraction: Defatted sample was soaked in 0.5M acetic acid containing pepsin (20kU/g, Sigma-Aldrich Corp., St. Louis, MO) with a sample solution ratio of 1:6 (w/v) for 48hours, and the filtrate was collected for precipitation.19
Two-step acid and one-step pepsin extraction: Collagen was extracted with 0.5 acetic acid with the solid/solvent ratio of 1:6 (w/v) for 24hours. The residue was re-extracted with 0.5M acetic acid containing pepsin (20kU/g) at a solid/solvent ratio of 1:6 (w/v) at 4°C for 24hours. The filtrates were collected and combined for precipitation.21
Collagen precipitation
2.6M NaCl was used to precipitate collagen in the presence of 0.05M Tris (hydroxymethyl) aminomethane (pH 7.0). The precipitates were collected by centrifuging at 20,000xg for 60min at 4°C. The pellets were dissolved in 0.5M acetic acid and samples were dialyzed against ten volumes of 0.1M acetic acid and distilled water.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
The method developed by Nalinanon et al.,21 was used to perform SDS-PAGE with modifications. Each collagen extract (0.2mg) was dissolved in a 0.02M sodium phosphate buffer (100) containing 1% SDS with 3.5M urea (pH 7.2) and centrifuged at 8,500 x g for 5min at room temperature. Solubilized collagen samples were mixed with the sample buffer containing 0.05M Tris-HCl, 4% SDS and 20% glycerol at a sample/solution ratio of 1:1 and the samples were boiled in water for 12min. After cooling down, samples were loaded into a gradient SDS-PAGE gel (4%-20%) and subjected to electrophoresis using a mini protein ll unit (Bio-Rad Laboratories Inc., Richmond, CA) at 125V, 3A, 300W for 2hours. The gel was then stained with sypro ruby stains. The molecular weights of collagen extracts were estimated with a molecular-weight marker (Sigma- Aldrich Corp., St. Louis, MO) and were also compared with acid hydrolyzed type I calf skin collagen.
Statistical analysis
The data was analyzed using a Complete Randomized Design (CRD) and the Student’s t-test. CRD was performed for the collagen yield comparison study and two replicates were used for the analysis. The Student’s t-test was used to compare the yields in pepsin treatments. The differences were considered significant when P value was less than 0.05.
Yield comparison
Five different extraction procedures were conducted to extract rooster collagen to compare yield differences for each treatment method. The collagen yield was calculated as a percentage of the dry weight of the initial weight of the skin. Average collagen yields (Table 1) for two replicates of acetic acid, citric acid, NaOH, one-step acetic acid one-step pepsin, and two-step acetic acid one-step pepsin extractions for the rooster skin were 6.5, 5.7, 6.8, 36.2, and 37.5 percent dry weight, respectively. The mean collagen yields for pepsin extraction methods were statistically different than traditional extraction methods at the significance level at P<0.05. The methods employed for pepsin extraction after pre-swelling rooster skin with acetic acid gave higher yields than traditional methods with acetic acid, citric acid, and NaOH. However, there were no significant difference in yields obtained from one-step acetic acid and one-step pepsin and two-step acetic acid and one-pepsin extraction methods. In addition, as an advantage, the rooster skin collagen extractions were light in color and had no objectionable odor.
Extraction methods |
Replicate 1 (% Yield) |
Replicate 2 (% Yield) |
Average* ± SD (% Yield) |
Acetic acid |
6.6 |
6.3 |
6.45a ± 0.2 |
Citric acid |
5.9 |
5.5 |
5.7a ± 0.3 |
NaoH |
7.1 |
6.5 |
6.8a ± 0.4 |
One-Step Acid One-Step Pepsin |
36.26 |
36.1 |
36.18b ± 0.1 |
Two-Step Acid One-Step Pepsin |
37.8 |
37.1 |
37.45b ± 0.5 |
Table 1 Yield comparison of rooster collagen extracts for different extraction methods
The rooster skin was completely digested in pepsin extraction procedures in the presence of acetic acid and gave much higher yields than the traditional methods which did not give complete digestion of the skin. These results suggested that pepsin with acetic acid can break more bonds between collagen than the other traditional methods. This observation for pepsin digestion was in agreement with Nagai & Nalinanon et al.,20,21 who obtained higher collagen yields with pepsin extractions than traditional extraction methods. However, these results were in disagreement with Morimura et al.,19 who had higher collagen yields for acetic acid (72%) and alkali (80%) extractions for collagen that was extracted from defatted pig skin. Further, the two-step acetic acid one-step pepsin did not have a significant increase in yield even though it took 24hours more processing time than one-step acetic acid one-step pepsin extraction method. This result was in agreement with Munasinghe et al.,22 who suggested that the collagen extraction with one-step acetic acid and one-step pepsin could be the most appropriate method in terms of the time and the expenses involved with collagen extraction for industrial uses.
However, rooster collagen yields obtained from each extraction methods were less than those of broiler skin collagen yields, 6.1, 6.2, 5.0, 38.7, and 40.4% respectively, as was shown in Munasinghe et al.22 The difficulties in recovery of rooster skin samples during the pretreatments and filtration steps were lead to give a lower collagen yields for rooster skin than that from broiler chicken skin.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
The collagen extracts from all five extraction methods were analyzed using the SDA-PAGE. The first lane of the image (Figure 1) had the molecular weight marker and the second lane had calf skin collagen. Lanes 3, 4, 5, 6, 7 and 8 were acetic acid, citric acid, NaOH, acetic acid, two-step acetic acid and one-step pepsin, and one-step acetic acid and one-step pepsin extractions, respectively. The old extraction methods, acetic acid, citric acid, and NaOH extractions, had bands that were more than 100kD molecular weights and lower molecular weights were observed in pepsin-solubilized collagen (less than 66kD). There were no differences in relative mobility of acid-solubilized rooster collagen and calf skin type 1 collagen.
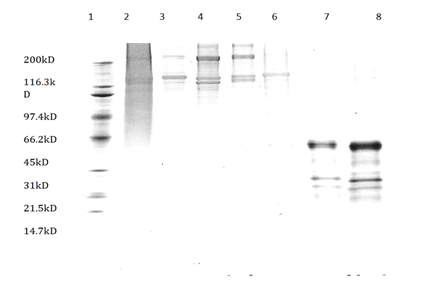
Figure 1 SDS-PAGE protein patterns with sypro ruby stain (1) Molecular weight marker, (2) Calf skin collagen, (3) Acetic acid-solubilized collagen, (4) Citric acid solubilized collagen, (5) Akaline-solubilized collagen, (6) Acetic acid-solubilizes collagen (7) Two-step acid one step pepsin solubilized collagen (8) One step acid one step pepsin solubilized collagen.
These results were in agreement with the findings by Nalinanon et al.,21 who showed that there were no differences in the relative mobility of high molecular weights between acid extracted collagen from bigeye snapper skin and calf skin type I collagen. The higher molecular weights in old extraction procedures suggest that cross-links did not fully digest by acetic acid, citric acid, and NaOH extraction procedures. The lower molecular weights (less than 66kD) for pepsin extracted collagen in Lane 7 and 8 suggest that pepsin digests more peptide bonds than that with acid and alkali treatments. These results in agreement with Nalinaanon et al.,21 who found lower molecular weights in pepsin extracted collagen compared to acid extracted collagen and speculated that the high molecular-weight components might be degraded to smaller components by the pepsin action. Miller et al.,9 also suggested that intra-molecular cross-links were broken with pepsin digestion resulting in lower molecular weights.
The electrophoretic patterns of collagen extracts were analyzed in a non-reducing environment in the absence of β-mercaptoethanol as it has been shown that there were no differences in the electrophoretic patterns with or without β-mercaptoethanol in acid solubilized collagen and pepsin solubilized collagen with skin of Brownstripe red snapper & Nalinanon et al.,21,25 also found that collagen does not contain any disulfide bonds.
Several studies indicated that age and available feed could influence the collagen structure. For example, Foegeding & Sikorski et al.,1,26 showed that starving fish had more cross-linked collagen than that of well-fed fish. Foegeding et al.,26 showed that the high molecular weight cross-linked molecules in collagen increased with animal age. The rate of cross-link formation in collagen may be different between species. For example, the cross-linking rate of fish collagen may be different than that in rooster collagen. Sikorski et al.,1 showed that the bands were thick and mechanically stronger in starving fish than that in the fish that were adequately nourished. Roosters may have more cross-linked molecules in collagen because of their longer life spans than those of broilers, which have shorter life and are well-fed with nutrients. Therefore, rooster collagen may be mechanically stronger than the collagen obtained from well-fed broilers.27
Collagen was extracted from rooster skin using five different extraction procedures: acetic acid, citric acid, NaOH, one-step acetic acid and one-step pepsin and two-step acetic acid and one-step pepsin extractions. minced rooster skin was pretreated to remove non-collagenous protein, mineral, and fat. The removal of non-collagenous protein and mineral from rooster skin took 6 and 24hours, respectively, and the removal of thick fat layers in rooster skin took 48hours. Average collagen yields for collagen extracts with acetic acid, citric acid, NaOH, one-step acid one-step pepsin and two-step acid one-step pepsin were 6.45, 5.7, 6.8, 36.18, 37.45% dry weight, respectively. Higher yields could be obtained from the pepsin digestion and the efficiency in collagen extraction could be enhanced by incorporating pepsin at 20k units/g with acetic acid. These results indicated that the pepsin could digest more inter and intra molecular cross-links than traditional methods. However, pepsin-solubilized collagens showed lower molecular weights than those of the older extraction methods. Acetic acid, citric acid, and alkali extracted collagen had molecular weight over 100kDa and pepsin digestions had less than 66kDa. Further, rooster collagen extracts were light in color and had no objectionable odor to disguise.
This research suggests that the rooster collagen can be utilized as an alternate source of collagen for many industries including the food industry. Rooster collagen may be mechanically stronger than other collagen sources such as broilers that have shorter life spans than roosters.
This work was supported by Evans-Allen project. The authors would like to thank Dr. Kuwamy Nyame and Mr. Matthew Whittiker at University of Maryland Eastern Shore for their technical assistance. The authors specially thank Banuja Munasinghe for assisting them with lab work. The authors also are grateful to Drs. Christopher Briand and Alexander Halperin at Salisbury University for reviewing this article.
The author declares no conflict of interest.

©2017 Munasinghe, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.