Journal of
eISSN: 2373-4310


Research Article Volume 7 Issue 1
Food Technology Research Institute, Egypt
Correspondence: Abdelazim SAA, Food Technology Research Institute, Agriculture Research Center, 9EL GAMMAA ST GIZA, Egypt, Tel +201113810090
Received: March 26, 2017 | Published: August 24, 2017
Citation: Abdelazim SAA, Masoud MRM, Youssif MR. Micronutrients for natural carbonated and noncarbonated soft drink. J Nutr Health Food Eng. 2017;7(1):204-212. DOI: 10.15406/jnhfe.2017.07.00226
Micronutrients (minerals, vitamins, organic acids and phenolic compounds), Physical, chemical characteristics and microbiological evaluation for natural carbonated and noncarbonated soft drink (anise, cinnamon, fennel and ginger) were determined. The overall acceptability values were a reflection of all the tested quality attributes and acceptability of the studied carbonated and noncarbonated soft drinks. The results demonstrated that, there were no significant differences (p <0.05) between (anise and fennel) or (cinnamon and ginger) carbonated soft. Generally the obtained results for organoleptic evaluation of carbonated soft drink and noncarbonated made from anise, cinnamon, ginger and fennel extracts indicated that there were differences between carbonated and noncarbonated soft drink, carbonated had high overall acceptability values than noncarbonated soft drink. The results of physical, chemical and microbiological evaluation characteristics of carbonated and non-carbonated soft drinks made from anise, cinnamon, ginger and fennel extracts had the same recorded. The general observation from the micro-biological analysis shows that as the medium becomes more acidic (pH and titratable evaluation) the concentration of micro-organism was reduced. It could be noticed that the no significant amount ofminerals contained anise, cinnamon, ginger and fennel carbonated and noncarbonated soft drinks. On the other hand, anise carbonated and noncarbonated soft drinks showed the highest Iron, Zinc and Magnesium content (3.98±0.01, 2.50±0.01 and 64.90±0.01mg/100ml, respectively). However, cinnamon extract recorded the highest concentration of Manganese (9.62±0.02mg/100ml). It could conclude that the anise, cinnamon, ginger and fennel carbonated and noncarbonated soft drinks are rich sources for many compounds such as minerals, vitamins, organic acids and phenolic compounds.
Keywords: soft drinks, carbonated, medicinal plants, minerals, vitamin, organic acids, phenolic compounds
HFCS, high fructose corn syrup; UV, ultraviolet; 5-HMF, 5-hydroxy methyl furfural; NEB, non-enzymatic browning; TPC, total plate count; LSD, least significant differences
Beverages are produced in almost every country in the world and their availability is remarkable. From the largest cities to some of the remotest villages, soft drinks are available in a variety of flavours and packaging. Carbonated beverages constitute the major part of the world wide soft drinks industry. The market for these products also continues to show a remarkable potential for growth.1
Carbonated Soft Drinks are the beverages with added carbon dioxide that gives an effervescent taste to the beverages. Carbonated soft drinks are further divided into colas and non-colas, as well as diet and regular soft drinks. The cola-flavoured carbonated beverages usually contain added phosphoric acid as acidulant because it can strengthen the acidity. Phosphoric acid has the same characteristics as the cola flavours, which are dry and sometimes balsamic.2,3 Cola soft drinks use cola nut from Cola nitida and Cola acuminata trees of Africa as their flavour agent. Non-cola soft drinks usually use citric acid as acidulant.4
Non-carbonated soft drinks are soft drinks without carbon dioxide and sparkling taste and they include fruit punch, fruit drinks, ice tea, coffee with sugar, and sport drinks. Non-carbonated soft drinks do not undergo the carbonation process and do not have any sparkling flavour. The sugar used to sweeten the regular soft drinks is either sucrose or high fructose corn syrup. Regular soft drinks have approximately the same amount of sugar as a glass of pineapple or orange juice, 7-14g/100ml. Diet soft drinks use aspartame, saccharine, acesulfam K, or sucralose as their sweetener soft drink is slightly acidic in order to give pleasant tartness to the product and preserve it. The most common acidulated in soft drinks are citric acid and phosphoric acid.5
Furthermore, Troiano & Schamm6,7 the beverages are in the top ten contributing foods for several nutrients, included carbohydrates, vitamins,minerals as well as energy, especially to children. The phosphorus content in cola type carbonated beverages could have reduced levels of the active form of vitamin D and led to a decline in calcium absorption and to bone decalcification, increasing bone fracture risk. The calcium: phosphorus ratio is a significant risk factor for bone fractures.8,9 All cola beverages contain 40 to 70mg phosphorus per 12Oz serving.10
More recently, Low and Alhuthali suggest that the increasing weight loss in tooth enamel during dental erosion in soft drinks can be attributed to the continuous leaching of calcium ions, in addition to phosphorus. Daniela et al.,11 Assessed the erosive potential of a light cola drink when compared to a regular one in situ/ex vivo and suggested that the light cola drink is less erosive than the regular one.
The contributions of soft drink Intake to obesity were studied. Soft drink intake was identified as one of the contributory factors to obesity or weight gain.12 The energy given by a sugar content of beverage was more preserved by the body than a mix nutrient beverage of the equal volume and energy content because these kinds of beverages had a smaller thermic effect.13 There was also a positive relationship between sugar-sweetened drink intakes and both greater weight gain and risk of type 2 diabetes.14 Soft drinks contained high fructose corn syrup and glucose that could enhance the energy over consumption. The high fructose corn syrup (HFCS) intake enhancement was related temporally to the obesity epidemic, and too much HFCS in soft drinks might have a role in the obesity epidemic.
A soft drink is slightly acidic in order to give pleasant tartness to the product and preserve it. The most common acidulants in soft drinks are citric acid and phosphoric acid.5
The main objective of the present investigation is to study the use of natural carbonated and noncarbonated soft drink as sources of micronutrients.
Materials
Medicinal plants: Four types of medicinal plants were selected to carry out this study. The plants selected are Anis fruits (Pimpinella anisum L), Cinnamon (Cinnamomum verum Presl, Syn), Fennel fruits (Foeniculum vulgare) and ginger rhizome (Zingiber officinale Roscoe). The required quantities of the above-mentioned plants were purchased from the local market at Fayoum governorate, Egypt. The samples were packaged in plastic bags and stored at low temperature until used.
Chemicals: The chemicals that used in chemical analysis and identification of phenolic compounds and organic acids were obtained from Sigma Chemical Company (St. Louis, Mo 63178, USA). The materials used in preparing the microbiological media were obtained from Biolifes, Milano, Italy, and Carbon dioxide gas, Sugar, were purchased from the Company of Sugar and Integrated Industries at El-Hawamdia, Giza governorate, Egypt.
Carbonator (Carbonation machine): The machine that used in the carbonation process was purchased from OPM Bois Briand, Nantes Cedex, Paris, France.
Bottles: Transparent plastic bottles of 150ml capacity equipped with screw caps were used in filling and bottling the beverages prepared. The bottles were obtained from the local market at Cairo governorate, Egypt.
Experiments
Preparation of medicinal plants extracts: Each sample of the medicinal plants was properly sorted and cleaned to remove the defects and the undesirable particles. It was then carefully crushed using mill (Moline machinery LTD, model No. 352-4, Germany) before extraction. The extraction was carried out using hot water. The mixture of raw material and water was boiled for 10min, followed by straining through muslin cloth to remove the plant particles. The filtered solution was allowed to stand for about 30min to enable any sediment to be settled and removed. The clear solution was carefully decanted to obtain a clear extract of the medicinal plant. The extracts were stored at low temperature until using in the preparation of carbonated beverage.
Preparation of carbonated beverages: The pure medicinal plant extract was boiled for 5min to eliminate microorganisms followed by filtration. The extract was then blended with sugar at a level of about 10 °Brix and its pH value was adjusted to about 3.0 using citric acid. Afterwards the beverage was carbonated by injection of CO2 gas. The carbonation process was carried out according to the method described by Abdelazim,1 according to Figure 1 carbonate the beverage it was the first pre-chilled in a refrigerator to decrease the temperature to about 2-4 °C to facilitate the absorption of CO2 gas which was injected in the extract with the aid of the carbonation machine (Carbonator). The products of carbonated soft drinks were then bottled and capped immediately to maintain their gas contents. The beverages were sensory evaluated and analyzed for their chemical characteristics.
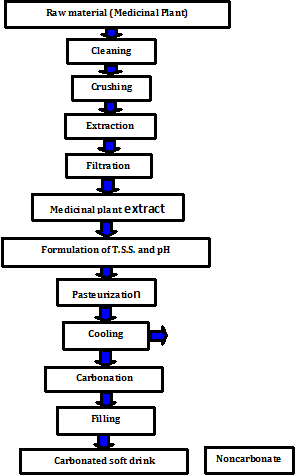
Figure 1 Flow sheet for the production of carbonated and noncarbonated soft drink from medicinal plant.
Methodology
The following analytical methods were used for the analysis of raw materials of medicinal plants and the carbonated soft drinks prepared from the medicinal plant extracts. The samples of beverages were degassed prior to analysis to remove all the gas dissolved.
Sensory evaluation: The organoleptic properties of the carbonated and noncarbonated beverages produced from the medicinal plants were evaluated according to Shachman7 and modified by Abdelazim1 to indicate the consumer acceptability of the products. The quality attributes evaluated were color, flavor, taste, sweetness fizzy and the overall acceptability of the carbonated and noncarbonated soft drinks. Ten panelists participated in the evaluation. The panelists were presented all the coded samples simultaneously and they were requested to show their preferences for the quality attributes using a numerical scale of 1-10. The score values were statically analyzed according to the method of Snedecor and Cochran.15
Physical and chemical characteristics determination: Total soluble solids (°Brix), pH, Total Titratable acidity (as % anhydrous citric acid), specific gravity and Moisture of carbonated soft drink determined according.16
Determination ofminerals : Ash content was measured by calculations, overnight at 550ºC in a furnace, with constant mass.17 Sodium, potassium, magnesium, iron, calcium, Chloride, Copper, Iodine, phosphorus and zinc were determined using atomic absorption spectrophotometer (Perkin Elmer model 3300, Merck hybrid system USA).
Determination of vitamins: Vitamin A (retinol) was measured as described by Leth and Jacobsen18 using Bechman HPLC, Vitamin B1 (Thiamine) and vitamin B2 (riboflavin) were measured by HPLC as described by Bogner and Vitamins C contents were determined by HPLC according to the method of Romeu N et al.,19 other vitamins were determined according to the.17
Color index: The color of anise, cinnamon, ginger and fennel beverages were determined calorimetrically as described by Ranganna.16 The eighty mole of the beverage was diluted to 100ml of acetone. The diluted sample was filtered and Absorbance was measured in the filtrate at a wavelength of 420nm. The Spectronic 20, Spectrophotometer, Bush and Lamb were used in color determinations.
Clarity: The clarity of carbonated and noncarbonated soft drink was measured according to the method described by Endo.20 Beverage sample was centrifuged at 5000rpm for 30min and the transmittance (T %) of the supernatant obtained was measured at 660nm using Spectronic 20, Spectrophotometer, Bush and Lamb.
Gas volume: The volume of carbon dioxide gas in the carbonated soft drink samples was measured according to the method described by Shachman.7
Phenolic compounds: Phenolic compounds were determined by HPLC according to the method of Goupy et al.21 The final products of carbonated and noncarbonated soft drinks were filtered through a 0.2µm Millipore membrane filter than 1-3ml was collected in a vial for injection into HPLC Hewllet Packared (series 1050) equipped with an auto sampling injector, solvent degasser, ultraviolet (UV) detector set at 280nm and quarter HP pump (series1050). The column temperature was maintained at 35°C. Gradient separation was carried out with methanol and acetonitrile as a mobile phase at a flow rate of 1ml/min. Phenolic acid standard from sigma Co. Were dissolved in a mobile phase and injected into the HPLC. Retention time and peak area were used for calculation of phenolic compound concentration in the data analysis of HEWLLET Packared software.
Organic acids: The concentration of each of the organic acids: citric, fumaric, lactic, malic, oxalic and tartaric acid were determined by HPLC according to the method of Wodecki et al.22 The final products of carbonated and noncarbonated soft drinks filtered only through a 0.2µm Millipore membrane filter than 1-3ml was collected in a vial for injection into HPLC Hewllet Packared (series 1050) equipped with autosamplling injector, solvent degasser, ultraviolet (UV) detector set at 210 NM and quarter HP pump (series1050). The column temperature was maintained at 55°C. An isocratic separation was carried out with water containing 0.1% o-phosphoric acid as a mobile phase at a flow rate of 1ml/min. The organic acids standards (citric, fumaric, lactic, malic, oxalic and tartaric acids) from sigma Co. Were dissolved in a mobile phase and injected into the HPLC. Retention times and peak area were used to calculate the concentration of organic acids by the data analysis of HEWLLET Packared software.
Microbiological evaluation of carbonated and noncarbonated soft drinks: Total Plate Count (TPC) and Mold and yeast counts methods described by Merck23 were used for detecting the safety value of carbonated and noncarbonated soft drinks during storage. Oneml of each beverage was withdrawn under aseptic conditions and transferred into a sterile tube contained 9ml of sterilized distilled water.
Statistical analysis: Data collected for chemical analysis and sensory evaluation were statistically analyzed by the least significant differences (LSD) at the 5% level of probability procedure according to the method of Snedecor & Cochran.15
Sensory evaluation of carbonated and noncarbonated soft drinks made from medicinal plant extracts
Herbal extracts, especially the infusion type, can be incorporated into soft drinks,mineral water-based drinks and energy drinks. On account of theirmind-calming and soporific properties anise, cinnamon, ginger and fennel are the herbs used in Egypt water-based drinks.
Carbonated soft drinks are widely consumed. A good portion of their appeal, however, comes from the promotion of the products in a way that is attractive to younger customers.24 Carbonated water constitutes up to 94% of a soft drink. Carbon dioxide adds that special sparkle and bites to the beverage and also acts as a mild preservative. Carbon dioxide is a uniquely suitable gas for soft drinks because it is inert, non-toxic, and relatively inexpensive and easy to liquefy.25
The organoleptic properties of carbonated and noncarbonated soft drinks made from anise, cinnamon, ginger and fennel water extracts. The carbonated and noncarbonated soft drinks were evaluated by ten panelists for color, flavor, taste, sweetness and overall acceptability as shown in Tables 1 & 2. Soft drinks may be served chilled or at room temperature.25
Carbonated Soft Drink |
Color (10) |
Flavor (10) |
Taste (10) |
Sweetness (10) |
Overall Acceptability (10) |
Category* |
Anise |
8.5b± 0.63 |
9.7a± 0.94 |
9.9a± 1.30 |
9.9a± 0.73 |
9.6a± 0.69 |
Excellent |
Cinnamon |
9.45a± 1.33 |
8.6b± 0.94 |
8.7b± 0.87 |
8.0b± 0.66 |
8.7b± 0.87 |
Very good |
Fennel |
9.20a± 1.52 |
9.5a± 1.41 |
8.8a± 1.71 |
7.8bc± 1.39 |
9.6a± 0.68 |
Excellent |
Ginger |
8.35b± 1.33 |
8.5b± 0.94 |
8.6b± 0.80 |
8.0b± 0.66 |
8.7b± 0.82 |
Very good |
LSD |
0.71 |
0.69 |
0.51 |
0.76 |
0.67 |
Table 1 Mean score values for organoleptic evaluation of carbonated soft drink (CSD) made from anise, cinnamon, ginger and fennel extracts
* Category: 9 ≥ Excellent, 8 ≥Very good, 7 ≥ Good, 6 ≥ Fair, 5and ≤ = Poor
**Any two means, at the column have the same litter did not significantly different at the 5.0 % level of probability.
Non-Carbonated Soft Drink |
Color (10) |
Flavor (10) |
Taste (10) |
Sweetness (10) |
Overall Acceptability (10) |
Category* |
Anise |
7.25a±1. 33 |
9.0a±0. 94 |
8.4c±1.30 |
8.0b±0.66 |
8.7b±0.87 |
Very good |
Cinnamon |
7.40b±0.63 |
8.0b±0.94 |
8.2e±1.30 |
8.9a±0. 73 |
8.5c±0.91 |
Very good |
Fennel |
7.40b±0.63 |
9.0a±0. 94 |
8.3c±1.30 |
8.9a±0. 73 |
8.6b±0.91 |
Very good |
Ginger |
7.00c±0.53 |
8.0b±0.94 |
8.1e±1.30 |
9.4a±0. 69 |
8.5c±0.73 |
Very good |
LSD |
0.61 |
0.59 |
0.51 |
0.66 |
0.57 |
Table 2 Mean score values for organoleptic evaluation of non-carbonated soft drink (CSD) made from anise, cinnamon, ginger and fennel extracts
* Category: 9 ≥ Excellent, 8 ≥Very good, 7 ≥ Good, 6 ≥ Fair, 5and ≤ = Poor
**Any two means, at the column have the same litter did not significantly different at the 5.0 % level of probability.
Color is considered an important indicator of quality, usually being the first sensory attributes observed by the consumer. In addition, tonality and the intensity of the color can provide information about the quality of the raw material used in its preparation.26
The results in this table showed that, there were no significant differences (p<0.05) in color between the cinnamon and fennel soft drinks. On the other hand, significant differences (p<0.05) in color between the anise and ginger soft drinks were recorded.
Concerning the flavor, no significant difference (p <0.05) was recorded between cinnamon, fennel and ginger soft drink, but there were significant differences (p<0.05) between anise and the all other soft drinks. Aurelia and Cristian25 mentioned that, the overall flavor of a soft drink depends on an intricate balance of sweetness, tartness, and acidity (pH).
For taste, carbonated soft drink the obtained results in a Table 1 indicated that there were no significant differences (p<0.05) between anise and fennel carbonated soft drinks, also there were no significant differences (p<0.05) between cinnamon and ginger carbonated soft drinks. On the other hand, there were significant differences (p <0.05) between (anise and fennel) and (cinnamon or ginger) carbonated soft drinks. Generally there were significant differences (p<0.05) between anise, fennel, cinnamon and ginger carbonated and noncarbonated.
Sweetness there were significant difference (p<0.05) with anise and all samples carbonated and noncarbonated.
The overall acceptability values were a reflection of all the tested quality attributes and acceptability of the studied carbonated and noncarbonated soft drinks. The results demonstrated that, there were no significant differences (p <0.05) between anise and fennel and also no significant cinnamon and ginger carbonated or noncarbonated soft.
Generally the obtained results for organoleptic evaluation of carbonated soft drink and noncarbonated made from anise, cinnamon, ginger and fennel extracts in Table 1 & 2 indicated that there were differences between carbonated and noncarbonated soft drink, carbonated had high overall acceptability values than non carbonated soft drink.
Physicochemical properties and microbiological analysis of carbonated and noncarbonated soft drinks made from anise, cinnamon, ginger and fennel extracts
The results in Table 3 which shows the ˚Brix, pH, acidity, total solid, specific gravity, color, gas volume and microbial count of carbonated and noncarbonated soft drinks made from anise, cinnamon, ginger and fennel extracts. Total solid is the amount of dry material remaining after all the water is evaporated.27 The T.S.S of anise, cinnamon, ginger and fennel extracts were recorded 10.00˚Brix this value according to El-Faki AE et al.28
Character |
Anise |
Cinnamon |
Ginger |
Fennel |
|
Total Soluble Solids (°Brix) |
10.0±0.05 |
10.0±0.01 |
10.0±0.02 |
10.0±0.03 |
|
Total Titratable acidity (%) * |
0.31±0.01 |
0.40±0.01 |
0.32±0.01 |
0.31±0.01 |
|
°Brix / Acid ratio |
32.25±0.5 |
25.00±0.3 |
31.25±0.4 |
32.25±0.1 |
|
pH |
3.13±0.02 |
3.01±0.03 |
3.03±0.01 |
3.04±0.01 |
|
Color index** |
0.347±0.01 |
0.420±0.01 |
0.420±0.01 |
0.270±0.01 |
|
Clarity (T% at 660 nm) |
99.4±0.5 |
99.2±0.2 |
98.9±0.6 |
99.6±0.5 |
|
Gas volume*** |
3.50±0.02 |
3.50±0.01 |
3.50±0.11 |
3.50±0.12 |
|
Specific gravity at 20 °C |
0.6782 |
0.6783 |
0.6783 |
0.6783 |
|
Bacterial count (cells/ml) |
Carbonated |
2±0.01 |
3±0.01 |
1±0.01 |
2±0.02 |
Noncarbonated |
3±0.01 |
4±0.01 |
3±0.01 |
4±0.02 |
|
Molds and yeasts (cells/ml) |
Carbonated |
2±0.02 |
3±0.01 |
2±0.02 |
2±0.01 |
Noncarbonated |
3±0.02 |
4±0.01 |
4±0.02 |
3±0.01 |
|
Table 3 Physical and chemical characteristics and microbiological analysis of carbonated and noncarbonated soft drinks made from anise, cinnamon, ginger and fennel extracts
*Determined as citric acid. **Absorbance at A420 nm for anise, cinnamon and fennel beverages
*** Gas volume of carbonated soft drinks
The pH values of anise, cinnamon, ginger and fennel extracts were 3.13±0.02, 3.01±0.03, 3.03±0.01 and 3.04±0.01 respectively. Though the pH values were relatively similar which can be attributed to the proximity of the total titratable acidity as citric acid of all the samples (0.31±0.01 to 0.40±0.01%) with no significant difference? According to Kaanane et al., 29 the minimal change in pH can be explained by relationship existing between pH and free acid content. The high pH of a food is used as an indicator of bacterial spoilage (i.e. the food with a high pH is more susceptible to microbial spoilage).
The amount of mineral dissolved during the erosion depends on several factors included the pH, the buffering effect, and the length of the exposure time. The buffering effect is the concentration of acids in the beverages or the ability of an acidic solution to keep its pH unaffected in dissolving enamel apatite and diluting with saliva. The stronger the buffering effect, the more mineral will be dissolved before the equilibrium pH can be reached and stop the dissolution. However, the presence of a certain quantity of calcium, phosphate and fluoride in the drinks may have a protective effect against the dissolution.30,31
Non-enzymatic browning (NEB) is one of the most important chemical reactions responsible for quality and color changes during the heating or prolonged storage leading to brown coloration, due to chemical reactions such as caramelization, ascorbic acid degradation and the Millard reaction. It is the most common quality problem and causes loss of nutrients and the formation of intermediate undesirable compounds like furfural and 5-hydroxymethylfurfural (5-HMF). Data in the same table showed that color index (non-enzymatic browning) of anise, cinnamon, ginger and fennel extracts were 0.34±0.015, 0.42±0.011, 0.42±0.011 and 0.27±0.012 respectively. Concerning to the clarity of the soft drinks made from anise, cinnamon, ginger and fennel extracts the obtained results indicated that all soft drinks were record high values for the clarity being 99.4±0.05, 99.2±0.2, 98.9±0.06 and 99.6±0.5% respectively.
Also, all the above mentioned carbonated soft drinks made from anise, cinnamon, ginger and fennel extracts had recorded the same gas volume being 3.50. The specific gravity of all the samples anise (0.6782±8.4×10-5), cinnamon (0.6783±8.4×10-5), ginger (0.6783±8.4×10-5) and fennel (0.6783±8.4×10-5) showed no significant difference. This was in line with the amount stated for all beverages, non-alcoholic (including soft drinks and juices) and fruit drinks (low calories and undiluted).32
Results in the same table shows microbiological analysis of the final product of the bacteria count, both of cinnamon and fennel samples have the highest count of (3±0.58, 5±0.53and 3±0.52, 5±0.52cell/ml, carbonated and noncarbonated respectively probably because of its low acidic content which caused a decrease in the bacteria count. Yeast and mold count were observed for the carbonated and noncarbonated soft drinks made from anise, cinnamon, ginger and fennel extracts. Carbonated and noncarbonated anise and ginger soft drinks had the lowest bacteria, yeast and mold count of 1±0.40, 3±0.40and 2±0.42, 3±0.42 cell/ml, carbonated and noncarbonated soft drinks respectively. This could be attributed to the high acidic medium which inhibits the growth of micro-organisms.
Also, ginger significantly inhibits the growth of both gram-positive and gram-negative bacteria.33 In the same way Beuchat34 mentioned that, fennel inhibits the growth of pathogenic and toxin-producing microorganisms in food. The general observation from the micro-biological analysis shows that as the medium becomes more acidic (pH and titratable evaluation) the concentration of micro-organism was reduced. The gram-negative strains of bacteria, especially E. coli, have less sensitivity to fennel essential oil Cantore et al.35 Fennel essential oils exhibit an inhibitory effect against a wide range of Bacillius species Ozcan et al.,36 Mimica-Dukic et al.,37 Mimica-Dukic N et al.,38 reported that the essential oils of fennel are active against Aspergillus species .Ozcan et al.,36 reported the antifungal activity of essential oils of bitter fennel as expected, fennel seed extracts offered antimicrobial activity.
Minerals content: From the results presented in Table 4, it could be noticed that, both of anise, cinnamon, ginger and fennel extracts contained a significant amount of important minerals. The potassium concentration ranged from 149.00±0.02 to 320.70±0.00mg/100ml for fennel and anise. Potassium is an essential nutrient and has an important role is the synthesis of amino acids and proteins.39 Cinnamon extract showed the highest concentration of calcium (75.36±0.02mg/100ml) followed by anise extract (71.70±0.01mg/100ml) and ginger extract (70.66±0.01mg/100ml). While, fennel extract recorded the highest concentration of sodium (45.55±0.02mg/100ml). Magnesium is an essential mineral for enzyme activity, like calcium and chloride; magnesium also plays a role in regulating the acid-alkaline balance in the body. Phosphorus is needed for bone growth, kidney function and cell growth. It also plays a role in maintaining the body’s acid-alkaline balance.40 On the other hand, anise extract showed the highest Iron, Zinc and Magnesium content (3.98±0.01, 2.50±0.01and 64.90±0.01mg/100ml, respectively). However, cinnamon extract recorded the highest concentration of Manganese (9.62±0.02mg/100ml).
Minerals |
Mineral Composition of Carbonated Soft Drink (Mg/100ml) |
|||
|---|---|---|---|---|
Anise |
Cinnamon |
Ginger |
Fennel |
|
Iron (Fe) |
3.98±0.01 |
1.20±0.01 |
1.36±0.00 |
3.52±0.011 |
Zinc (Zn) |
2.50±0.01 |
1.66±0.011 |
1.85±0.00 |
1.40±0.00 |
Manganese (Mn) |
0.85±0.02 |
9.62±0.02 |
8.55±0.01 |
0.98±0.02 |
Magnesium (Mg) |
64.90±0.01 |
19.70±0.01 |
17.98±0.02 |
41.03±0.01 |
Sodium (Na) |
42.15±0.01 |
15.67±0.01 |
17.66±0.00 |
45.55±0.02 |
Calcium (Ca) |
71.70±0.01 |
75.36±0.02 |
70.66±0.01 |
66.20±0.02 |
Potassium (K) |
320.70±0.0 |
156.37±0.01 |
166.37±0.01 |
149.00±0.00 |
Table 4 Mineral composition of carbonated and noncarbonated soft drinks made from anise, cinnamon, ginger and fennel extracts
Our results on minerals are on par with the earlier report on medicinal plant species41 where in mg, K, and Ca were found to be pre-dominant. Belitz et al.,42 have reported that the dietary intake of essential minerals should be >50mg/day. The essential minerals: Ca, K and mg are important in extracellular and intracellular body functions and as components in the building blocks of structural components in the human body. Also, the presence of high levels of potassium in diets is considered to be beneficial for those suffering from hypertension and excessive excretion of potassium through the body fluids. minerals like iron and selenium, even if present in threshold levels can act as antioxidants and are involved in strengthening the immune system. Whereas, magnesium and zinc are known to prevent cardiomyopathy, muscle degeneration, growth retardation, dermatitis, gonadal atrophy, impaired spermatogenesis, congenital malformations and bleeding disorders.41 It could conclude that carbonated soft drinks made from the medicinal plants are rich sources for much of the valuable minerals that are essential and required for humans.1
Vitamins content: There were significant differences in the ascorbic acid (vitamin C) contents of the carbonated and non-carbonated soft drinks made from anise, cinnamon, ginger and fennel extracts. From the obtained results, it could be showed that fennel extract recorded the highest value (380.60mg/100ml) of ascorbic acid followed by anise extract (344.00mg/100ml), ginger extract (225.35mg/100ml) and cinnamon extract (221.00mg/100ml) respectively. Since ascorbic acid is soluble in water, it is readily lost via leaching from cut or bruised surfaces of raw material; however, in processed foods the most significant losses result from chemical degradation.43 Transformation of ascorbic acid to diketoglutaric acid due to reaction with air and metal ions may also contribute to the losses encountered.44 From the same Table, it could be seen that ginger extract had higher values of vitamins B2 and B12 being 0.289 and 0.294mg/100ml, respectively, followed by a cinnamon extract which recorded 0.284 and 0.185mg/100ml, respectively. While, fennel extract was recorded the lower values of vitamins B2 and B12 being 0.029 and 0.128mg/100ml, respectively.
Concerning to riboflavin content, the obtained results indicated that cinnamon extract was recorded the higher content followed by fennel, anise and ginger extracts which amounted 0.294, 0.275, 0.241 and 0.240mg/100ml, respectively.
For pyridoxine content, the obtained results indicated that fennel extract was recorded the higher content followed by anise, ginger and cinnamon extracts which amounted 0.037, 0.033, 0.026 and 0.014mg/100ml, respectively.
On the other hand, anise extract was recorded the higher content of nicotinic acid followed by ginger, cinnamon and fennel extracts being 0.426, 0.179, 0.115 and 0.076mg/100ml, respectively.
Results in the same table show that, fennel extract was recorded the higher content of folic acid followed by ginger, cinnamon and anise extracts being 229.934, 186.098, 176.137 and 152.525mg/100ml, respectively Table 5.
Vitamins |
Vitamins Contents (Mg/100ml) |
||||||
|---|---|---|---|---|---|---|---|
Vit.C |
Thiamin (B1) |
Cobalamins (B12) |
Riboflavin (B2) |
Pyridoxine (B6) |
Nicotinic acid (B3) |
Folic acid (B9) |
|
Anise |
344 |
0.073 |
0.171 |
0.241 |
0.033 |
0.426 |
152.525 |
Cinnamon |
221 |
0.284 |
0.185 |
0.294 |
0.014 |
0.115 |
176.137 |
Ginger |
225.35 |
0.289 |
0.294 |
0.24 |
0.026 |
0.179 |
186.098 |
Fennel |
380.6 |
0.029 |
0.128 |
0.275 |
0.037 |
0.076 |
229.934 |
Table 5 Vitamin C and vitamins B contents of carbonated and noncarbonated soft drinks made from anise, cinnamon, ginger and fennel extracts
Phenolic compounds content: Phenolics are plant compounds that have many different functions, including adding color to the plant and attracting bees and other insects for pollination.45 Many phenolics appear to have anticancer and antioxidant effects in humans.46,47 The total concentrations of each group of phenolic compounds in the studied seeds are listed in Table 6. Catechein, P. Hydroxy benzoic and Salicylic were the major free phenolic compounds existed in anise extract (503, 120 and 371mg/100ml, respectively). While in ginger extract, Caffeic (27mg/100ml), Vanillic (22mg/100ml) and Ferulic (26mg/100ml) were the predominant phenolic compounds. Catechein was the main phenolic compound in Cinnamon extract, representing about 392mg/100ml, followed by Salicylic (248mg/100ml), P. Hydroxy benzoic (64mg/100ml), and Caffeine (48mg/100ml). On the other hand, the major phenolic compounds content in fennel extract were Salicylic (314mg/100ml), Catechein (290mg/100ml), P. Hydroxy benzoic (24mg/100ml) and Ferulic (22mg/100ml) Abdelazim1 reported that the beverages contents of many phenolic substances that possess significant antioxidant capacities make the carbonated beverages made from medicinal plants associated with several health functions such as adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides Table 6.
Phenolic Compound |
Concentration of Phenolic Compounds (Mg/100 Ml) |
|||
|---|---|---|---|---|
Anise |
Cinnamon |
Ginger |
Fennel |
|
Catechein |
503 |
392 |
380 |
290 |
P. Hydroxy benzoic |
120 |
64 |
73 |
24 |
Chlorogenic |
31 |
24 |
26 |
15 |
Caffeic |
10 |
25 |
27 |
11 |
Syringic |
26 |
16 |
18 |
4 |
Caffeine |
63 |
48 |
50 |
11 |
Vanillic |
18 |
20 |
22 |
15 |
Ferulic |
11 |
23 |
26 |
22 |
P. Coumaric |
4 |
9 |
10 |
6 |
Salicylic |
371 |
248 |
256 |
314 |
Cinnamic |
10 |
45 |
20 |
10 |
Total |
1167 |
914 |
908 |
722 |
Table 6 Phenolic compounds contents of carbonated and noncarbonated soft drinks made from anise, cinnamon and fennel extracts
Organic acid content: Results presented in Table 7 show the changes in organic acids content (i.e. Citric, Formic, Tartaric, Malic, Oxalic and Lactic) of carbonated and noncarbonated soft drinks made from anise, cinnamon, ginger and fennel extracts. From the present data, it is clearly noticed that anise extract contained the higher value in citric (0.779±0.001), Fumaric (0.790±0.002), tartaric (0.348±0.001), malic (0.622±0.003) and lactic (0.038±0.002) than all other soft drinks made from cinnamon, ginger and fennel extracts. Whereas both of ginger and fennel extracts contained the higher value in oxalic (0.043±0.001).
Organic Acids |
Concentration of Organic Acids (Mg/100 Ml) |
|||
|---|---|---|---|---|
Anise |
Cinnamon |
Ginger |
Fennel |
|
Citric |
0.799±0.001 |
0.137±0.003 |
0.127±0.001 |
0.205±0.003 |
Fumaric |
0.790±0.002 |
0.087±0.001 |
0.077±0.003 |
0.104±0.001 |
Tartaric |
0.348±0.001 |
0.026±0.002 |
0.036±0.001 |
0.079±0.003 |
Malic |
0.622±0.003 |
0.116±0.001 |
0.126±0.003 |
0.168±0.002 |
Oxalic |
0.039±0.001 |
0.033±0.002 |
0.043±0.002 |
0.043±0.003 |
Lactic |
0.038±0.002 |
0.033±0.001 |
0.022±0.001 |
0.030±0.002 |
Table 7 Organic acid contents of carbonated and noncarbonated soft drinks made from anise, cinnamon, ginger and fennel extracts
From the obtained data, it could be observed that, cinnamon contain 0.137±0.003, 0.087±0.001, 0.026±0.002, 0.116±0.001, 0.033±0.002 and 0.033±0.001mg/100ml for citric, fumaric, tartaric, malic, oxalic and lactic acids, respectively. Concerning to organic acid contents of carbonated and noncarbonated soft drinks made from ginger extract, the obtained results show that ginger extract content citric, fumaric, tartaric, malic, oxalic and lactic acids were recorded 0.127±0.001, 0.077±0.003, 0.036±0.001, 0.126±0.003, 0.043±0.002 and 0.022±0.001mg/100ml, respectively. For fennel, the obtained results indicated that fennel extract was recorded 0.205±0.003, 0.104±0.001, 0.079±0.003, 0.168±0.002, 0.043±0.003and 0.030±0.002mg/100ml for citric, fumaric, tartaric, malic, oxalic and lactic acids, respectively. The organic acids found in beverages made from the medicinal plants are of importance from both healths related and manufacturing perspectives.1
It could conclude that the carbonated and noncarbonated soft drinks can made from anise, cinnamon, ginger and fennel extracts. The obtained results for organoleptic evaluation of carbonated soft drink and noncarbonated made from anise, cinnamon, ginger and fennel extracts indicated that the carbonated had higher overall acceptability values than noncarbonated soft drink. They are rich sources of some compounds (minerals, vitamin, organic acids and phenolic compounds) that are essential and required for humans. The study also confirmed the good microbiological stability of carbonated and noncarbonated soft drinks made from anise, cinnamon, ginger and fennel extracts. We are suggested in the future studies on the effect of storage carbonated and noncarbonated soft drink.
I wish to express my sincere gratitude and thanks to Dr. Khalil Ebraheim Khalil and Dr. Awad Abdel Tawab Mahomoud, Food Science and Technology Department, Faculty of Agriculture, Fayoum University, and crops Technology Research Department, Food Technology Research Institute (FTRI), Agriculture Research- Center-Giza
Author declares that there is no conflict of interest

©2017 Abdelazim, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.