Journal of
eISSN: 2373-4310


Research Article Volume 5 Issue 3
1Department of Food Science and Human Nutrition, Colorado State University, USA
2Department of Psychology, Colorado State University, USA
3Department of Nutrition and Dietetics, University of Northern Colorado, USA
Correspondence: Mary A. Harris, PhD, RDN, Colorado State University, Department of Food Science and Human Nutrition, 234 Gifford Building, Campus Delivery 1571, Fort Collins, CO 80523-1571, United States, Tel +1 970 491 7462
Received: October 28, 2016 | Published: December 1, 2016
Citation: Abou-Dobara MI, Ismail MM, Refaat NM. Intake of total omega-3 docosahexaenoic acid associated with increased gestational length and improved cognitive performance at 1year of age. J Nutr Health Food Eng. 2016;5(3):642-651. DOI: 10.15406/jnhfe.2016.05.00176
Background: Maternal omega-3 docosahexaenoic acid (n-3 DHA) intake during pregnancy and/or lactation has been positively associated with infant growth and cognitive development. However, the majority of studies have not examined the effect of supplementing mothers with n-3 DHA during both pregnancy and lactation and fail to account for total maternal n-3 DHA intake.
Aims: To determine the effect of increasing n-3 DHA intake during pregnancy and lactation on infant neurocognitive development in the firstyear of life.
Study Design: A randomized, double-blinded, placebo-controlled design was used.
Subjects: 115 pregnant women were randomized to receive purified tuna oil supplement containing 300mg of n-3 DHA and 67mg of eicosapentaenoic acid (EPA) per day or an identical placebo (Sunola Oil) for the last trimester of pregnancy through the first 3months of lactation.
Outcome Measures: Neurocognitive development was measured using the Bayley Scales of Infant Development III at 4 and 12months of age. Gestational length was determined sing the LMP method.
Results:Infants born to mothers with >600mg n-3 DHA/day showed significantly higher scores on the 12month cognitive scale of the BSID-III (p=0.018) compared to infants born to mothers with <300mg n-3 DHA/day. Infants born to mothers in the n-3 DHA supplement group had an increase of 4.5days in gestational age (p=0.048) and significantly lower incidence of preterm birth (5%; n=3) compared to infants born to mothers in the control group (18%; n=10; c2=4.97, p=0.026).
Conclusions:Maternal intake of ≥600mg n-3 DHA/day during the third trimester of gestation throughout the first threemonths of breastfeeding was associated with small but significant increases in gestational length and enhanced neurocognitive performance in infants at 1year of age.
Clinical Trial Registration Number: NCT02219399 Clinical Trials.gov
Keywords: docosahexaenoic acid (n-3 DHA), infant, cognitive development, pregnancy, lactation, omega-3 (n-3) fatty acids
Omega-3 docosahexaenoic acid (n-3 DHA), a 22 carbon, six double bond omega-3 long chain polyunsaturated fatty acid (LCPUFA) is present in high concentrations in brain and neural tissue. Accumulation is greatest during the third trimester of gestation through the first 2years of life.1 During the last trimester, it is estimated the fetus acquires 67-75mg per day of n-3 DHA, accounting for 80% of brain n-3 DHA accumulation between week 26 and 40 of gestation.2,3 The fetus acquires n-3 DHA through placental transfer of n-3 DHA, which is influenced by n-3 DHA in the maternal diet, enhanced mobilization of n-3 DHA from maternal adipose stores, and maternal n-3 DHA synthesis.4 Synthesis of n-3 DHA from the essential dietary precursor alpha-linolenic acid (ALA) occurs in humans, but the conversion is estimated to be only <0.1-1%.5,6 Women of child-bearing age may convert ALA to n-3 DHA more effectively due to the proposed influence of estrogen on n-3 DHA synthesis, yet the conversion may be insufficient to meet needs during periods of rapid growth and development.6 Low conversion rates suggest a requirement for preformed n-3 DHA obtained directly from the diet or supplements, during pregnancy and lactation. While no current daily U.S. recommended intake exists for n-3 DHA, the European Influence of Dietary Fatty Acids on the Pathophysiology of Intrauterine Foetal Growth and Neonatal Development (PERILIP) group recommends pregnant and lactating women receive at least 200mg per day of preformed n-3 DHA, through supplementation or consuming one to two portions of fatty fish high in n-3 LCPUFA per week.7
Studies have demonstrated numerous benefits of maternal fish and seafood8‒13 intake and/or n-3 DHA supplementation14‒22 during pregnancy, including increased gestational age, decreased preterm birth incidence, and improved infant growth and cognitive development. The large Avon Longitudinal Study of Parents and Children demonstrated that women consuming greater than 340g of fish per week gave birth to children with decreased risk for poor performance on age appropriate tests of cognitive development compared to mothers with low seafood intake during pregnancy.12 Postnatal n-3 DHA supplementation studies have shown no conclusive effect of DHA intake on infant growth and neuro cognitive development and, when significant, the most dramatic effects are seen in preterm infants.23‒28 These studies have been extensively reviewed and results are currently inconclusive.29‒32 Contrasting results from n-3 DHA trials may be due differences in timing or exposure as well as type and dose of n-3 DHA supplementation. Another possible explanation is that total n-3 DHA exposure (total intake from food and supplemental sources) has not been accounted for. The present study was designed to examine the effects of n-3 DHA intake during pregnancy and lactation on infant neuro cognitive development using the BSID-III during the first year of life.
Study design
115 women with singleton pregnancies were recruited from private practice gynecology and obstetrics clinics in the Fort Collins, Colorado between 24-28weeks of gestation. Inclusion criteria were: age 18-42years, singleton pregnancies and willingness to breastfeed exclusively for the first 3months of life. Exclusion criteria included maternal age <18years, multiple fetuses, diabetes, HIV positive status, chronic illnesses or other conditions which could preclude breastfeeding and any known allergies to seafood or fish oils. The Institutional Review Board at Colorado State University approved all protocols and procedures. All women provided written informed consent prior to participation.
Women were randomized to receive a supplement of highly purified tuna fish oil containing 300mg n-3 DHA and 67mg EPA provided as Hi-DHA™ tuna oil (Nu-Mega Oil, Clover Corporation, Ltd) prepared as one hard capsule or an identical Sunola™ a high oleic acid sunflower oil placebo. The dose was selected based on previous pilot data with a power of 0.05% at the 80% confidence level. A computer generated block randomization procedure was used to assign women to supplemental groups. Study supplements were provided in opaque bottles and participants were instructed to keep supplements in the provided container in a freezer. Breastfeeding support using a successful peer coaching program33,34 was provided to promote exclusive breastfeeding for the first three months of life. During this period, maternal supplementation was maintained to provide post-natal exposure from breast milk consistent with relative exposure levels during fetal development.
A food frequency questionnaire (FFQ) previously validated against erythrocyte n-3 DHA22 and revalidated in this study population was used to estimate total n-3 DHA intake from food, prenatal vitamins and other sources. The FFQ included sources of n-3 DHA intake from fish and seafood, n-3 DHA enriched food products such as eggs or milk, and any fish oil or n-3 DHA supplements. Information was quantitated using the United States Department of Agriculture (USDA) National Nutrient Database for Standard Reference, Release 22. Total weekly and daily n-3 DHA intake from the FFQ was calculated and verified with a 7-day diet record data. The 7-day diet record information was analyzed using Nutritionist Pro (Axxya Systems, Stafford, TX) software and total daily n-3 DHA intake from food was calculated. Sociodemographic data, the date of the last menstrual period (LMP) and estimated due date were collected. Self-reported pre-pregnancy weight and height was used to determine pre-pregnancy body mass index (BMI).
Sample collection and fatty acid analyses
A baseline blood sample was collected from women during their oral glucose screen between 24-28weeks of gestation and stored briefly at 4oC until separated. Blood samples were collected in a Vacutainer containing EDTA and separated by centrifugation within 24hours into erythrocyte, plasma and buffy coat fractions and stored at -80oC until further analysis. Breast milk samples were obtained at 2 and 4months postpartum and expressed using manual or electric breast pumps into sterile collection cups and transported on ice to the laboratory and immediately frozen and stored at -20oC. Lipids were extracted from maternal erythrocytes, plasma, and 2 and 4month breast milk samples using a modified Folch extraction (chloroform:methanol 2:1 v/v). Plasma phospholipids were further separated by thin layer chromatography and all lipid extracts were resuspended in 0.5ml chromatographic grade hexanes. Fatty acid methyl esters (FAME) were prepared+ by direct transmethylation using 14% boron trifluoride in methanol (Sigma Chemical Co, St. Louis, MO). FAME were analyzed via gas chromatography on an Agilent 6890 chromatograph, using a ramped temperature program and flame ionization detection and a J&W DB225 column. Individual fatty acids were identified by comparison of retention times with known FAME.
Infant cognitive testing
The BSID-III Mental Development Index (MDI) subscales were administered to infants at 4 and 12months of age by a trained technical assistant overseen by a licensed clinical psychologist in the Psychology Department at Colorado State University and used as the primary study outcome. The BSID-III was selected as a measurement of neurocognitive development due to its ease of administration, test-retest reliability and for comparison to previous studies. The BSID-III MDI cognitive, language, social-emotional and general adaptive behavior scales were completed. To control for confounding effects of maternal IQ, mothers completed a maternal IQ test using the abbreviated Wechsler Adult Intelligence Scale (WAIS), including the block design and vocabulary subtests, which are highly correlated with full scale IQ.35 To control for the confounding effect the degree of stimulation in the home environment may have on cognitive development, the mothers completed a self-administered Home Screening Questionnaire (HSQ) at 9months of age.36
Gestational length
Gestational length was calculated indays using the LMP method and infant date of birth, as a secondary outcome. Infant anthropometric data was obtained from pediatric medical records.
Data analysis
Participating women, all data collectors and investigators were blinded to supplement allocation until all study children were 12months of age and had completed the cognitive testing. After all study data was collected, the study was un-blinded only to study investigators for analysis. Power was based on a previous pilot trial determining the effect n-3 DHA supplementation during pregnancy has on improved infant BSID scores at 4-6months, 32 infants per supplementation group are necessary to reach a power of 0.05% at the 80% confidence level. Total daily n-3 DHA intake was calculated by adding estimated dietary n-3 DHA intakes, maternal n-3 DHA supplementation (if applicable) and n-3 DHA study supplement. Data were analyzed based on supplement group, placebo or n-3 DHA, as well as daily n-3 DHA divided into three intake groups: low = 0-299mg per day n-3 DHA, medium=300-599mg per day n-3 DHA, high=≥600mg per day n-3 DHA. Cut-off points for the intake groups were determined on the basis of those falling below, at the median recommended intake and above the currently recommended intake.37,38
Statistical analysis was completed using SPSS statistical software (SPSS, Chicago, IL) and GraphPad Prism V4.0 (GraphPad Software, San Diego, CA). Results are expressed as means±SD, unless otherwise specified. Two-tailed t test was used to analyze results based upon supplement group and one-way ANOVA was used to compare means between all daily n-3 DHA intake groups. Tukey’s post hoc tests were conducted on all statistically significant ANOVA results to determine if differences existed among group means. Correlations were computed using Pearson’s correlation coefficient. Data was adjusted for potential confounders, when appropriate, including, maternal age at delivery, maternal BMI, maternal IQ, breastfeeding duration, gestational age and the effects of the home environment on cognitive development via the HSQ.
Of the 115 women initially included in the study, 55 were randomly assigned to the placebo and 60 to the n-3 DHA supplement. Study completion rates were 80% in the n-3 DHA supplemented group but only 64% in the placebo oil group. In both groups, 90% of the women whom completed the study consumed 99% of their supplements. Demographic data, including age, maternal weight, maternal BMI, maternal IQ and percent n-3 DHA in erythrocytes and at 2months post-partum did not differ between women that completed the study and those that did not.
Complete demographic data for each group (by supplement group and by total n-3 intake) are provided in Table 1. There were no significant differences between the placebo and n-3 DHA supplemented groups, nor among any of the intake groups, with respect to maternal age, race, marital status, pre-pregnancy maternal weight and height, or maternal IQ. The mean maternal age at delivery was 31.5±4.4years of age, 93% were Caucasian and 93% were married. All enrolled pregnant women are accounted for, as shown in the consort diagram in Figure 1, with 90% completing the 4month BSID cognitive testing, and 71% completing the 12month cognitive testing. Additionally, 81% of enrolled women were still breastfeeding at 2months, 78% were still breastfeeding at 4months, and 57% had continued to breastfeed until study completion at 12months. There was no significant difference in overall breastfeeding duration between supplement groups or DHA intake groups.
|
|
Treatment |
Daily DHA Intake |
|||||
|---|---|---|---|---|---|---|---|
|
Placebo |
DHA |
Pc |
Low DHA (0-299mg DHA/d) |
Medium DHA (300-599mg DHA/d) |
High DHA (>600mg DHA/d) |
Pd |
|
|
No of participants |
55 |
60 |
27 |
54 |
34 |
||
|
Maternal age(y) |
31.2±4. .4b |
31.7±4.4 |
0.513 |
30.2±4.9 |
31.8±4.4 |
32.0±3.7 |
0.203 |
|
Maternal Race [n(%)] |
|||||||
|
African American |
0(0) |
1(1.67) |
0(0) |
1(1.9) |
0(0) |
||
|
Caucasian |
52(95) |
55(92) |
26(96) |
47(87) |
34(100) |
||
|
Hispanic |
2(3) |
2(3) |
1(4) |
3(5.5) |
0(0) |
||
|
Asian |
1(2) |
1(1.67) |
0(0) |
2(3.7) |
0(0) |
||
|
Other |
0(0) |
1(1.67) |
0(0) |
1(1.9) |
0(0) |
||
|
Maternal weight(kg) |
66.0±10.9 |
67.7±15.8 |
0.495 |
65.9±10.4 |
69.1±16.5 |
64.2±10.5 |
0.236 |
|
Maternal height(cm) |
166.4±5.5 |
165.2±67 |
0.309 |
167.0±4.9 |
164.5±6.2 |
166.9±6.8 |
0.105 |
|
Maternal BMI(kg/m^2) |
23.8±3.8 |
24.9±6.1 |
0.27 |
23.6±4.0 |
25.7±6.2 |
23.0±3.4 |
0.040e |
|
Marital Status [n(%)] |
|||||||
|
Married |
51(93) |
56(93) |
25(93) |
48(89) |
33(97) |
||
|
Committed relationship |
4(7) |
1(2) |
2(7) |
4(7.4) |
0(0) |
||
|
Single |
0(0) |
3(5) |
0(0) |
2(3.7) |
1(3) |
||
|
Maternal IQ - WAIS |
|||||||
|
Vocabulary |
42.8±7.2 |
43.7±6.7 |
0.579 |
40.4±8.7 |
44.1±6.3 |
44.1±6.1 |
0.188 |
|
Block Design |
52.2±9.1 |
49.0±10.6 |
0.164 |
53.7±6.7 |
48.5±10.6 |
51.0±10.7 |
0.199 |
|
Maternal RBC DHA(% total fatty acids) |
6.17±1.47 |
5.75±1.55 |
0.137 |
5.91±1.48 |
5.48±1.21 |
6.72±1.70 |
0.0007c |
|
Maternal plasma DHA(% total fatty acids) |
5.17±1.36 |
4.97±1.53 |
0.455 |
4.99±1.30 |
4.83±1.43 |
5.47±1.52 |
0.125 |
|
DHA study supplement [n(%)] |
0(0) |
60(100) |
0(0) |
38(70) |
23(68) |
||
|
Voluntary DHA/Fish oil supplement [n(%)] |
39(71) |
38(63) |
12(44) |
32(59) |
34(100) |
||
|
DHA intake from food(mg/d) |
101±95 |
101±103 |
0.999 |
68±49 |
78±67 |
165±137 |
<0.00010e |
|
DHA intake from voluntary additional supplements(mg/d) |
258±262 |
165±208 |
0,035e |
74±94 |
129±157 |
447±260 |
<0.0001e |
|
Total daily DHA intake(mg/d) |
359±280 |
566±238 |
<0.0001e |
142±83 |
418±82 |
815±186 |
<0.0001e |
|
Preterm birth - <37wks [n(%)] |
10(18) |
3(5) |
6(22) |
4(7) |
3(9) |
||
|
Late term birth - >40wks [n(%)] |
15(27) |
16(27) |
6(22) |
16(30) |
9(27) |
||
|
Cesarean Delivery [n(%)] |
12(22) |
20(33) |
5(19) |
17(31) |
10(29) |
||
|
Gestational length(d) |
274.4±14.8 |
278.9±7.8 |
0.048 |
272.8±16.4 |
277.4±11.6 |
278.9±7.8 |
0.135 |
|
Breastfeeding duration(weeks) |
39.3±18.9 |
41.6±15.7 |
0.496 |
36.7±19.8 |
40.6±17.0 |
46.8±12.5 |
0.063 |
|
2 month breast milk DHA(% fatty acid) |
0.40±0.45 |
0.62±0.43 |
0.017 |
0.26±0.42 |
0.51±0.42 |
0.71±0.44 |
0.003e |
Table 1 Participants characteristic by supplement group (treatment) and total daily n-3 DHA intakea
aWAIS, Wechsler Adult Intelligence Scale; DHA, docosahexaenoic acid
bMean±SD(all such values)
cMean differences between placebo and DHA supplemented groups were tested with two-tailed t test
dMean differences between daily DHA intake groups were assessed by ANOVA
eSignificant (p<0.05)
Daily total n-3 DHA intake ranged from 18mg to 1.374g per day which included the n-3 DHA supplement. Mean n-3 DHA intake from food of all enrolled subjects was 101±99mg per day, from additional supplementation was 209±239mg per day, and total n-3 DHA intake of all women was 468±278mg per day. Analysis of 7-day diet records demonstrated no significant differences in macronutrient consumption, ALA, n-3 DHA, or fish intake at 2months among the women. Total daily n-3 DHA intake at 2months positively correlated with daily n-3 DHA intake at baseline (r=0.58, p<0.0001), indicating daily n-3 DHA intake of women remained similar from baseline through 2months.
Differences in placebo versus n-3 DHA supplement groups are shown in Table 1. There was no significant difference in n-3 DHA consumption from food, but total n-3 DHA intake per day was significantly higher in the n-3 DHA group compared to the control group (p<0.0001), indicating the study supplement did effectively increase n-3 DHA intake in the participating women. Maternal erythrocyte and plasma fatty acids percent total n-3 DHA at baseline were not significantly different at baseline between supplement groups. The mean percent total n-3 DHA in breast milk at 2months was significantly higher in the n-3 DHA supplement group compared to placebo control (p=0.017). After supplementation ended at 3months, breast milk percent total n-3 DHA levels remained significantly different at 4months (p=0.0007).
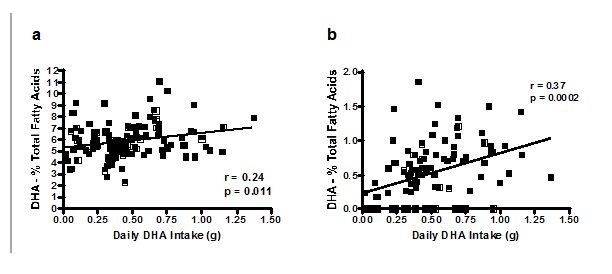
Of the 115 women, 27 women (23%) consumed less than 300mg total n-3 DHA per day and were classified in the low n-3 DHA intake group, 54 women (47%) consumed between 300 and 599mg n-3 DHA per day and were categorized in the medium n-3 DHA intake group, and 34 women (30%) consumed 600mg or more of n-3 DHA per day and were categorized in the high n-3 DHA intake group. DHA intake from food and voluntary supplementation was significantly different (p<0.0001 and p<0.0001, respectively) between n-3 DHA intake groups, with estimated intakes significantly higher in the high n-3 DHA intake group compared to both the low and the medium intake groups (Tukey’s HSD p<0.001). Total daily n-3 DHA intakes in the assigned intake groups were all significantly different from one another (p<0.001). Analysis of n-3 DHA status by daily DHA intake showed significantly higher erythrocyte % total n-3 DHA in women within the high daily n-3 DHA intake group compared to the medium intake group (Tukey’s HSD p<0.001). Erythrocyte % total n-3 DHA positively correlated with total daily DHA intake (r=0.24, p=0.011; Figure 3A). Percent total n-3 DHA in breast milk at 2months was significantly different among the total daily DHA intake groups (p=0.003), with higher n-3 DHA in the high intake group compared to the low n-3 DHA intake group (Tukey’s HSD p<0.01). Additionally, total daily n-3 DHA intake positively correlated with breast milk n-3 DHA proportion of fatty acids (r=0.37, p=0.0002; Figure 3B). Increases in daily n-3 DHA intake correlated with increases in breast milk n-3 DHA at 2months of breastfeeding. At 4months, after cessation of the study supplementation, breast milk percent total n-3 DHA levels remained significantly different (p<0.0001), perhaps due to voluntary continuation of n-3 supplements.
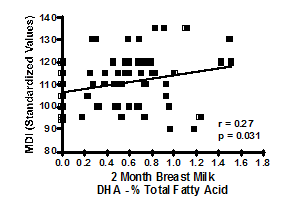
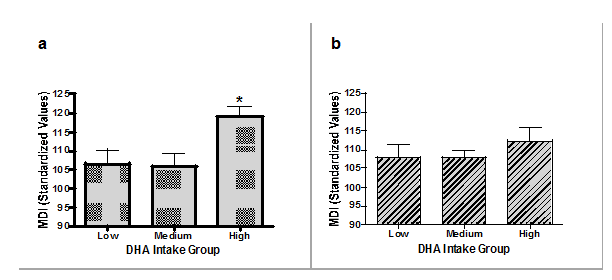
The results of the components of the 12month BSID-III MDI are presented both by supplement type and n-3 DHA intake group in Table 2. No statistically significant differences were found on any BSID-III scale at 4months of age, (data not shown). There were no statistically significant differences in the MDI scores at any time point, for any scale when analyzing by supplement group alone. Significant differences appear in the cognitive development of infants based upon maternal n-3 DHA intake group (p=0.018) at 12months of age. Infants born to mothers consuming greater than 600mg of n-3 DHA daily have higher scores on the 12month cognitive MDI test compared to both the low intake group and the medium intake group (Tukey’s HSD p<0.05). There was a positive correlation between breast milk % total n-3 DHA at 2months and 12month scores on the cognitive MDI scale (r=0.27, p=0.031), as exhibited in Figure 4. No significant differences were seen among any groups on infant 12month social and general adaptation scales. Furthermore, maternal age, IQ, breastfeeding duration or HSQ did not correlate with any of the BSID-III scores.
|
Treatment |
Daily DHA intake group |
||||||
|---|---|---|---|---|---|---|---|
|
Placebonn=35 |
DHA n=48 |
pc |
LowDHA (0-299mg DHA/d) n=17 |
Mediam DHA (300-599mg DHA/d) n=40 |
Heigh DHA (>600 DHA/d)mg n=26 |
pd |
|
|
Cognitive |
109.8±12.6b |
109.7±11.3 |
0.959 |
106.1±11.5 |
107.4±10.5 |
115.6±12.0 |
0.018e |
|
Language |
96.1±9.5 |
98.5±12.7 |
0.357 |
92.3±4.6 |
97.5±10.5 |
100.8±14.5 |
0.076 |
|
Social |
107.6±13.1 |
108.4±15.8 |
0.807 |
106.3±15,9 |
108.6±14.0 |
108.3±15,5 |
0.089 |
|
General Adaptation |
107.3±10.9 |
108.5±13.3 |
0.696 |
105.5±11.4 |
105.7±11.8 |
113.1±12.5 |
0.500 |
Table 2 Neurocognitive development at 12months of age measured by the Mental Development Index(MDI) of the Bayley Scale of Infant Development(BSID) III of infants born to mothers classified by maternal supplement group(treatment) and total daily n-3 DHA intakea
aDHA, docosahexaenoic acid
b Mean±SD(all such values)
cMean differences between placebo and DHA supplemented groups were tested with two-tailed t test
dMean differences between daily DHA intake groups were assessed by ANOVA
eSignificant(p<0.05)
Neurocognitive results were also analyzed based upon infant sex. There were no statistically significant differences comparing male and female infants for any of the 4 or 12month BSID-III tests. However, significant differences in the 12month MDI cognitive score of the BSID-III emerged in female infants based upon maternal daily n-3 DHA intake (p=0.010). Female infants born to mothers in the high n-3 DHA intake group scored 12.3 points higher on the 12month cognitive MDI scale (118.9±10.4) compared to the female infants born in the low intake group (106.6±12.9; Tukey’s HSD p<0.05) and scored 13 points higher compared to infants born in the medium total daily n-3 DHA intake group (105.9±12.7; Tukey’s HSD p<0.05). In contrast, no statistically significant differences were seen in male infants within n-3 DHA intake groups (p=0.529). There were no statistically significant differences between daily n-3 DHA intake groups for either gender on the social or general adaptation testing at 12months, as well as on all 4month BSID-III testing or when classifying by supplement group.
Gestational length in the n-3 DHA supplemented group was 4.5days longer than the placebo oil group (p=0.048). No statistically significant differences in gestational length were seen among total daily n-3 DHA intake groups, although there was a trend of 6.1 day longer gestational length in the high total daily n-3 DHA intake group compared to the low intake group (p=0.053). Total daily n-3 DHA intake positively correlated with gestational length (r=0.20, p=0.031), Figure 5. The incidence of preterm birth in the study was 11%, with a significantly greater incidence in the placebo oil group with 18% (n=10) compared to 5% (n=3; p=0.021) in the n-3 DHA supplemented group (c2=4.97, p=0.026). Total n-3 DHA intake during pregnancy was associated with a reduction in preterm births (OR= 4.2, 95% CI 1.10-16.26; RR=3.6, 95% CI 1.06-12.54). No statistically significant differences in the incidence of preterm birth were found comparing the total daily n-3 DHA intake groups. However, when combining the medium and high total daily n-3 DHA intake groups compared to the low daily n-3 DHA intake group, the incidence of preterm births significantly decreased from 22% (n=6) in the low daily n-3 DHA group to 8% (n=7) in women consuming greater than 300mg n-3 DHA per day (c2=4.87, p=0.027). Women consuming 300mg of n-3 DHA per day or greater had an associated reduction in the risk of preterm births compared to women less than 300mg n-3 DHA per day (OR= 0.3, 95% CI 0.09-0.92; RR=0.8, 95% CI 0.68-
Supplementing the diets of pregnant and breastfeeding women significantly increased total daily maternal n-3 DHA intakes. Pregnant women participating in the study had low baseline intakes of n-3 DHA, consuming roughly 90-100mg of n-3 DHA per day during pregnancy and lactation from food sources. These intakes agree with findings from previous studies investigating n-3 DHA intake in pregnant women.14,15,39 DHA from food provided 22% of the total daily n-3 DHA intake, indicating food sources of n-3 DHA impact total daily n-3 DHA have a significant impact on DHA exposure. While 67% of women consumed various other n-3 DHA (including prenatal vitamins), the addition of the n-3 supplement significantly increased total daily n-3 DHA intake by 58%. As demonstrated in this study, it may be important to account for total daily n-3 DHA intake in order to accurately evaluate the effect of maternal n-3 DHA on pregnancy outcomes.
It is fairly well established that n-3 DHA is important in fetal neurological development.40‒45 DHA is preferentially transported to the fetus during the last trimester of gestation, coinciding with fetal retinal and brain development, specifically with the growth spurt in gray matter and neurogenesis, which is maximal during the last trimester of gestation through two years of age.46 Results of numerous RCT showed no effects of n-3 DHA treatment during pregnancy, however, several RCT did show a significant correlation between maternal n-3 DHA status and infant neuro cognitive development.14,15,20 The absence of consistent correlations between n-3 DHA and infant neuro cognitive development in RCT could be due to the lack of accurately accounting for maternal n-3 DHA intake from all sources. The current study estimated total daily n-3 DHA intake from food and supplements throughout pregnancy and the first 3months of lactation, which allowed for the successful detection of a dosing effect of n-3 DHA intake on infant neuro cognitive development. The current study showed an association between maternal daily n-3 DHA intake of 600mg or greater and significantly improved scores on the cognitive MDI scale of the BSID-III at 12months of age. Similarly, a recent RCT that also accounted for daily n-3 DHA intake found infants born to women supplemented with a functional food containing 300mg n-3 DHA per day from the 24th week of gestation through delivery performed significantly better on problem-solving tasks at 9months of age compared to infants born to mothers of the placebo group.14
The timing and length of n-3 DHA supplementation are important factors in determining the effect n-3 DHA has on infant neuro cognitive development. Many previous RCT stopped n-3 DHA supplementation after birth, a time of extensive brain growth for the infant. Numerous studies have shown breastfeeding improves cognitive development of infants, and increased n-3 DHA content of breast milk is believed to be one of the major factors.25,47‒52 DHA is rapidly transferred into breast milk with no known selectivity and is altered within 2-3days based upon maternal intake of n-3 DHA.30,52 This study is in agreement with previous studies to show that n-3 DHA supplementation can successfully increase breast milk n-3 DHA which may play a role in continued infant cognitive development during this rapid stage of brain development. To date, only two other RCT supplemented n-3 DHA prenatally and maintained allocation to treatment postnatally.18,53 It is possible that the positive association between maternal n-3 DHA intake and persistence of improved infant cognitive development may require n-3 DHA supplementation during both pregnancy and lactation.
A topic of emerging interest is sex differences on n-3 DHA requirements, with boys and girls responding and benefiting differently to n-3 DHA supplementation. Through a stepwise regression analysis, the positive relationship between omega-3 fatty acid intake of 1 g or greater and cognitive test scores were found to be twice as strong in female American children in the Third National Health and Nutrition Examination Survey (NHANES III) compared to male children, suggesting female children may benefit more cognitively from increased omega-3 intakes.53 In the current study, female infants born to mothers in the high daily n-3 DHA intake group scored significantly higher on the 12month cognitive scale of the BSID-III MDI compared to female infants born to women in the low intake group. However, daily n-3 DHA intake did not significantly affect male cognitive test scores. Similarly, the DINO trial of early preterm infants did not find a significant effect of n-3 DHA on infant neuro cognitive development, yet, when focusing on sex differences, the study showed girls with higher n-3 DHA supplementation had significantly improved BSID MDI scores and a reduction in the risk for both mild and severe cognitive delays at 18months of age, with no observed affect in boys.27
Numerous RCT have attempted to quantitate the effect of n-3 DHA supplementation on increasing gestational length, while subsequently reducing the risk of preterm birth and the incidence of low birth weight infants.14,22,54,55 Several systematic reviews and meta-analyses have determined a slight (1.6 to a 4.5 day) increase in gestational length in women supplemented with n-3 DHA56‒58 and a decreased risk for preterm birth as high as 31% in high risk. The 4.5 day increase in gestational length and decrease in preterm birth seen in this study is in range with these estimates.
Mixed results of RCTs investigating neuro cognitive development may be attributable to differences in experimental design, including tine of exposure, length of supplementation, varying amounts of n-3 DHA and/or a failure to maintain assignment to treatment group postnatally. The current study attempted to resolve some of those issues which may account for the effects which were seen on gestational age and cognitive performance. The limitation of this study was that most of the significant effects were associational and not directly related randomized allocation and the lack of effect on the BSID-III at 4months of age. However, a previous study reported that 4month BSID scores did not significantly relate to later cognitive performance, but the 12month scores related significantly to preschool verbal and performance IQs as well as gross motor scores.59 Another limitation to this study was a completion rate of 80% in the n-3 DHA supplemented group compared to only a 64% completion rate in the placebo control group. Reasons for the differences between supplement group study completion rates are unknown. However, intent to treat analysis demonstrated that those who completed did not differ from those who completed on important variables predictive of infant cognitive development. Additionally, adherence to supplements excellent (>90%) in both supplement groups.
Many prenatal vitamins containing varying amounts of n-3 DHA have been introduced to the market within the last 10years and women choosing to take a n-3 DHA supplement were not excluded from the study. Awareness of possible benefits n-3 DHA and fish oil upon the developing fetus has increased over the last decade and more obstetricians and dietitians promote n-3 DHA supplementation during pregnancy and lactation. We noted that a large number of women were prescribed prenatal vitamins containing n-3 DHA or had chosen to take DHA or fish oil supplements, irrespective of assignment to study supplement group. Accounting for this more accurately estimated n-3 DHA exposure during pregnancy and may have been responsible for the positive associations found, suggesting that correcting for total n-3 DHA intake should not be ignored.
In conclusion, adequate maternal n-3 DHA intake from supplements and dietary sources may increase gestational length and enhance neuro cognitive development.
None.
Author declares that there is no conflict of interest.

©2016 Abou-Dobara, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.