Journal of
eISSN: 2373-4310


Research Article Volume 5 Issue 4
1Instituto Nacional de Investigaciones Agrícolas (INIA), Venezuela
2Instituto de Ciencia y Tecnología de Alimentos, Facultad de Ciencias, Universidad Central de Venezuela, Venezuela
3Escuela de Nutrición y Dietética, Facultad de Medicina, Universidad Central de Venezuela, Venezuela
4CIRAD, UMR Quali Sud, France
Correspondence: Clímaco Orlando Álvarez Fernández, Tapipa, Miranda state, Venezuela, Tel +58.0234-6621219
Received: October 26, 2016 | Published: December 6, 2016
Citation: Álvarez C, Pérez E, Lares MDC, et al. Identification of the volatile compounds in the roasting Venezuela criollo cocoa beans by gas chromatography- spectrometry mass. J Nutr Health Food Eng. 2016;5(4):659-666. DOI: 10.15406/jnhfe.2016.05.00178
An identification of the volatile compounds from the fermented dried and toasted (at 20°C, 25minutes) cocoa beans from a population of selected cocoa, were studied. The selected material "Criollo" (205 trees) was gathered from the plantation “El Pedregal” located at the south of Maracaibo Lake, Rio Frio " Mérida state, Venezuela. The volatile fraction was extracted and analyzed by gas chromatography coupled to a mass spectrometer (SPME/MS). The goal of the researching was the quantification of the content of organic volatile compounds developed by effect of the cocoa roasting. Volatile identification was performed comparing the retention indices and mass spectra obtained with those of the compounds existing as reference in the bank of the "Wiley Mass Spectral Data". Sixty-nine volatile compounds that were grouped into 12 chemical families were identified. Aldehydes, alcohols, acids, hydrocarbons, pyrazines and diverse compounds, were the compounds prevalent in the volatile fraction of roasted cocoa. As conclusion this research permits the establishment of the identification and sensory quality of the Venezuelan creole cocoa, is of consensus that the volatile profile of the Venezuelan cocoa, which is highly appreciated by the chocolatiers of the world, has been fewer studied. It is also observed that there is an effect of post-harvest treatment (fermentation, drying) and roasted on the formation of flavor compounds in the fraction of roasted cacao. The results of these studies also show that SPME has a significant advantage over more traditional methods for analysis of volatile compounds in the cocoa beans post-harvest process: no fermented, fermented, dried and roasted.
Keywords: cocoa, gas chromatography, mass, retention indices
Fermentation, drying and roasting are the most influential process in chocolate flavor formation.1,2 During fermentation a number of volatile precursors are developed due to the numerous enzymatic and chemical reactions convert to simple those complex compounds; such as: oligo and polysaccharides to mono saccharides or reducing sugars; and proteins to peptides and free amino acids.3 Studies have shown that unfermented cocoa beans do not contain the volatile precursors that are present in the fermented cocoa beans.4 On drying, the formation of these precursors it continues, and this compounds start to transforming into the volatiles that provide the chocolate flavor, that will be consolidate during the roasting process.5 Roasting is necessary to develop flavor, color, and volatile compounds of the chocolate. In roasting, these precursors participate in a series of biochemical reactions that occur inside of the grain, producing the characteristic chocolate "flavor".6‒11 Among them are included reactions of caramelization, "Maillard" and oxidation of polyphenols. As Davies & Labuzza,12 pointed out, the most important influence the Maillard reaction has on confectionery is the formation color, volatile and taste, mainly during processing, e.g. the flavor and color of many chocolate, candy and combination baked products. Thus, the Maillard reaction gives rise to the colored and the flavored compounds which are part of the food acceptance.13 The flavors produced during roasting come from a combination of 400-500 compounds.14 These compounds include aldehydes and pyrazines, which are formed during roasting, through the Maillard reaction and Strecker degradation of amino acids and sugars.15 Maillard reaction is a chemical reaction between amino acids and reducing sugars that gives browned food its desirable flavor. The reactive carbonyl group of the sugar reacts with the nucleophilic amino group of the amino acid, and forms a complex mixture of different low molecular weight characterized molecules responsible for a range of odors and flavors. This process is accelerated in an alkaline environment, as the amino groups (RNH3+) are deprotonated and, hence, have an increased nucleophilicity. The type of the amino acid determines the resulting flavour.16 Also the sugar choice has been shown to strongly influence the total amount of flavor formed, cited by Davies & Labuzza.12 The volatile fraction of the Venezuela Criollo type cocoa as a function of the postharvest treatment has been studied by Portillo et al.,17 and Álvarez C et al.,18 Both authors have emphasized the importance of the fermentation and drying on the final sensorial quality of the cocoa. Moreover, results are corroborating that the postharvest handling (fermentation and drying) contributes significantly in the expression and differentiation of volatile intensities among types of cocoa.19‒21 In Venezuela the postharvest handling is artesian, and is varying from a locality to another one. This factor added to the genetic contribution of the flavor precursor of the varieties of cocoa predicts differences in the final volatile profile of the cocoa or chocolate. This volatile composition neither is nor well studied in Venezuelan cocoa. The aim of this study was to identify by Gas Chromatography the volatile fraction of cocoa beans fermented, dried and roasted from a selected material of Criollo cacao trees of Venezuela.
Cocoa bean samples
Cocoa beans samples were obtained from a genetic representative population of 400 trees of the plantation “El Pedregal”, located in Mérida state (south of Lake Maracaibo), Venezuela. Samples were selected after 2years of morphological and molecular characterization22 from April to June crops in 2002 and 2003. Two hundred and five (205) trees from this population were randomly selected and harvested in order to study of the volatile fraction.
Sample preparation
Ripe pods were harvested (pruning shears, harvesting hook) from each tree one day before they were picked and prior to postharvest processing. To obtain approximately 800 and 880 g of dried cocoa beans, it was necessary to collect 2.2kg fresh cocoa seeds of each tree for a total of 14 to 15 mature and healthy pods, previously collected in the two periods of harvest (2002-2003). The pods were cut off by hand with a knife, and removing the beans from the rachis (central placenta). Then remove the pulp using saw dust or sand. Rinse 3 to 4times to eliminate the remaining sand or saw dust. Samples of cleaned beans were collected in a labelled container (plastic mesh bag) with a thickness of 4cm for posterior fermentation.
Postharvest treatment: Micro-fermentation
Figure 1 is a schematic representation of the procedure for the micro-fermentation of the samples in a 60x60x60cm squares wooden box (fermenter). (Figure 2A) (Figure 2B) are photos of the procedure on field.
The Figure 1 shown the ascendant order from bottom to top of the cocoa mass in the fermenter with a separation of 5cm between of fermenter wall sides, and covered with the rest of the cocoa mass. 2.2kg of fresh seed was placed for each placed for each flexible net bag. For the arrangement of the fermenter, the separation between the cocoa layer and the nylon mesh bags was 2-3cm. It was setting up inside the fermented with total of 16 nylon mesh bags, in four layer using a separation among them of 3cm of cocoa layer. The separation of the group of four bags from the wood walls of fermenter was 5cm. The top and the bottom of the fermenter were covered with a layer of cocoa mass of 15cm, and finally the fermenter was enclosed with a layer of banana leaves, and jute bags. The system only was turned it up at 24h, and the fermentation time was of 3 days. Once the fermentation was reached, it was preceded to drying it.
The fermented cocoa beans were sun-dried on a wooden floor. Each nylon mesh bag was open and spread out in a single layer on the floor (Figure 3). It was gently stirred 4-6times each day, for 4days, and by night it was covered with plastic sheet. Each sun-dried sample was then collected, placed in its marked plastic net bag and stored inside jute bags in a cool, dry and ventilated place.
All the samples were then sent to the laboratory CIRAD in Montpellier, France, for analysis. Once at CIRAD, the samples were roasted (120ºC; 25minutes). The fermented, sun dried and roasted beans were manually hulled, and milled with a pestle, until was reduced to 4-6mm of thickness. Fragments of nibs with 4-6mm of thickness were obtained. The nibs were packed and stored at -18ºC for further chemical analysis.
Extraction with SPME (Solid Phase Micro extraction)
2.85 grams of roasted nib cocoa were fragmented in a porcelain mortar and deposited into a 10mL vial with 50µl of butanol solution (Pattern Solution: 0.5 g.L-1) plus 3ml of double-distilled water. The vials, were hermetically closed with a butyl/Teflon septum using a specific sealer, leaving it until the equilibrium time be reach.
For the analysis, SPME fibres, covered with polydimethylsiloxane (PDMS) film (Supelco, Milan, Italy) with an outer diameter of 50/30 µm Carbowax/divinylbenzene (CWX) were used.
Before collecting a sample, the fibre was conditioned in the chromatograph injector at 270°C for 1hour and then it was exposed to sample/headspace, at 1 mm of distance from the interface between the fluid and headspace. Headspace equilibration time was at 50°C, 15min. For the determination of sample/headspace equilibration time, this parameter was ranged 15min to 45min, and constant temperature was fixed at 50°C.
Gas chromatographic analytical conditions
The gas chromatograph was an HP5890 Serie II equipped with FID and a 60mx0.32mmx0.25μm (DBWAX (J&W scientific) capillary column was used through all the work. The GC oven temperature was programmed from 40 to 180°C/min (heating rate of 2°Cmin-1). Helium (flow rate 1.5mLmin-1) was the carrier gas; and 20min run time. Temperature of the injector (operated on splitless mode) and of the detector was 250°C: the SPME fiber desorption time 4min. The injected sample volume was 1µL. Retention index (RI) was calculated by using n-alcanes as references.
Mass spectrometry (MS)
MS analyses were carried out with an HP Agilent series 6890 coupled to mass-spectrometer. Electron impact mass spectra were recorded at 70 eV (2.45 scans per second, 40 to 350uma (m/z)). The source temperature was 150°C to 260°C, line of transfer temperature. Spectral recording throughout elution was automatically performed with HP Agilent series 5973N software. MS chromatographic parameters are the same as the ones mentioned under Gas Chromatography.
Identification of the fraction volatile
Identification of the extracted analytes was done by comparison of the corresponding mass spectra in the GC-MS chromatograms with the data of "Wiley Mass Spectral Data" library.
Expression of results
To calculate the relative percentage (%) of each volatile compound the area normalization was performed using the following formula:
Ax: Area of each volatile component (X); Σi Ai: Sum of all areas (total area); Asp: standar pattern area; The relative percentage (%) were calculated using the standard (1-butanol) pattern as a reference.
Statistical evaluation of analytical data
Data collected of means, range and inferior and superior limits were performed with a n=205. Frequency Histograms was also calculated from the data.
%VC= ( Ax:Asp / ∑i Ai:Asp) × 100
Ax: Area of each volatile component (X); Σi Ai: Sum of all areas (total area); Asp: standar pattern area; The relative percentage (%) were calculated using the standard (1-butanol) pattern as a reference.
Statistical evaluation of analytical data
Data collected of means, range and inferior and superior limits were performed with a n=205. Frequency Histograms was also calculated from the data.
Analysis of volatile compounds
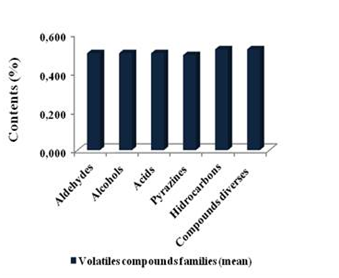
The chemical volatile fraction that were found in the population of Criollo cocoa (n= 205) was identified for a total of 69 volatile compounds, grouped into twelve (12) different chemical families (Table 1). In the same Table, the respective indexes and retention times (min) are shown. In general, the main volatile compounds identified were low molecular weight; such as, aldehydes, alcohols, ketones, esters, furans, sulfur compounds, pyrrols, pyrazines, and diverse compounds, which are typical of the flavor profile of roasted cocoa, in agreement with23,24 as it is shown in the Table I. Figure 4 is schematic representation of greatest amount of compound of the volatiletic fraction. Values of this main compound are: aldehydes (0.50%), alcohols (0.50%), acids (0.50%), hydrocarbons (0.52%), pyrazines (0.49%) and diverses compounds (0.51%) (Table 2).
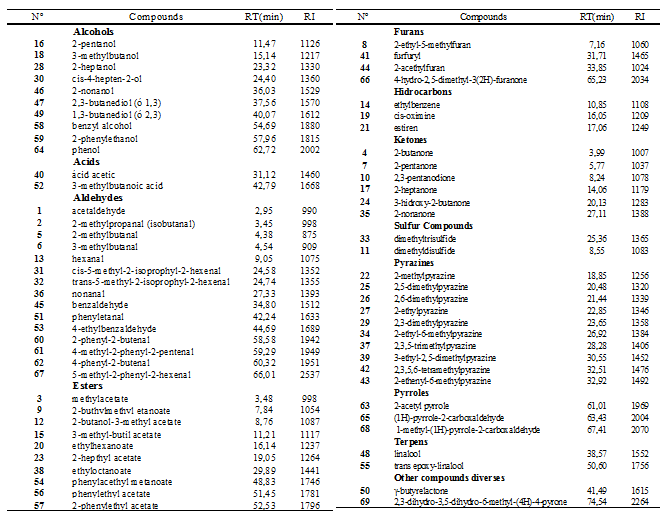
Table 1 Volatile compounds identified in the fermented, sun-dried and roasted beans of Criollo cocoa by GC-MS
Volatiles compounds Families (Total) |
Mean (%) |
SD |
Inferior Valeur (%) |
Superior Valeur (%) |
Aldehydes |
0.50 |
0.25 |
0.11 |
1.80 |
Alcohols |
0.50 |
0.28 |
0.16 |
1.44 |
Acids |
0.50 |
0.32 |
0.03 |
1.56 |
Ketones |
0.40 |
0.17 |
0.13 |
0.97 |
Esters |
0.48 |
0.27 |
0.11 |
1.49 |
Hidrocarbons |
0.52 |
0.50 |
0.02 |
1.83 |
Pyrazines |
0.49 |
0.31 |
0.02 |
1.75 |
Pyrrols |
0.47 |
0.24 |
0.03 |
1.24 |
Furans |
0.46 |
0.32 |
0.09 |
1.53 |
Sulfur compounds |
0.46 |
0.24 |
0.04 |
1.29 |
Terpens |
0.47 |
0.23 |
0.13 |
1.39 |
Compounds diverses |
0.55 |
0.26 |
0.06 |
1.49 |
Table 2 Average of volatiles content (%) for chemical families that are constituting the volatiletic fraction of the fermented, dried and roasted cocoa (n=205)
Frequency distribution of the main volatiles compounds in the Criollo cocoa population
The Figure 5 showed the frequency distribution of the volatile fraction of the population. The aldehydes content varied from 0, 10 to 1,11% with a frequency distribution of 98%, for most of the individual trees of the selected population. Quantitatively the values in the aldehydes content follow a normal Gaussian distribution (Figure 5A). The contents of alcohols and acids Figure 5B & 5C showed normal Gaussian distribution ranging from 0.12 to 1.32% and 0.20 to 1.62% respectively in the Criollo cocoa population. Pyrazines quantitatively constituted an important group after roasting. Frequency distribution of 98% with a mean of 0.50% varying from 0.10 to 1.30% had shown a normal distribution (Figure 5D). Pirazynes are typical roasting products and are generated only from well-dried beans cocoa.
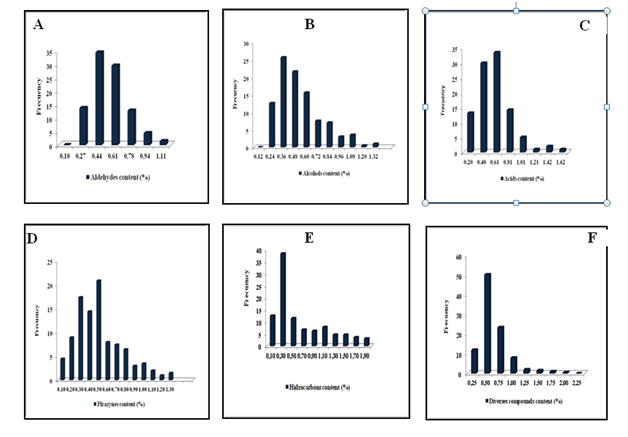
The hydrocarbons and diverse compounds represented an important fraction of the population Criollo cacao, with 95% and 97% of all trees tested respectively. The hydrocarbons frequency distribution showed 84% of trees with strong content ranging from 0.10 to 1.10% (Figure 5D). The 94% of the concentration in the distribution trees of the diverse compounds showed a content that ranged from 0.25 to 1.00 % and 6% with strong content 2.25 (Figue 5F).
Identification of the most relevant volatile compounds in population
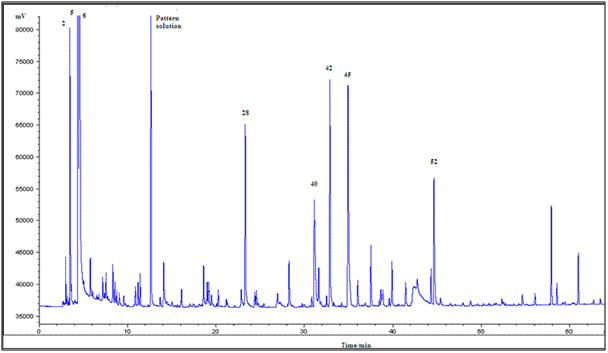
Figure 6 shown the typical GC-MS chromatograms peak profile of all samples studied. The most intense peak of the chromatogram corresponds to: 2-methylpropanal (2), 2-methylbutanal (5), 3-methylbutanal (6), and tetramethylpyrazine (42) (a product of the Maillard process), which was already recognized as one of the key odorants in cocoa products.25,26 The non-enzymatic browning or Maillard reactions are very important processes for the development of cocoa flavor, which primarily occur during the roasting process.27
The first flavor components result from two important processes: in fermentation e.g. the tetramethylpyrazine (42), which is produced by micro-organisms28 and during roasting, the formation of 3-methylbutanal, 2-methylbutanal and 2-methylpropanal, which are produced from the Strecker-degradation of free amino-acids.23
The tetramethylpyrazine proportionate the chocolate, cocoa, and coffee notes.25 Alkylpyrazines, also derived from Maillard reactions, emerge here as probably the most dominant and interesting volatiles compounds of dark chocolate.26 The concentration of all methylpyrazines varied with the temperature and the time of roasting.24
Other group are the alkyl pyrazines detected that are of less relevance, in they are included 2, 3, 5-trimethylpirazina (37) and the 2, 5-dimethylpirazine (25). All of them have a sensorial characteristic to cocoa flavor and roasted nut.
Probably, 2, 5-dimethylpyrazine (25), 2-ethylpyrazine (27), 2-acetyl pyrrole (63) and, furfuryl (41) provide praline and chocolate flavor. The presence of an ethyl group in the two pyrazine compounds suggests a key role for alanine and/or its Strecker aldehyde, acetaldehyde, in chocolate flavor synthesis.29,30 Sensory responses are not available for all pyrrols, 14 of which have been identified in cocoa products,27 however, 1-acetylpyrrole and, 2-acetylpyrrole (63) were described as astringent, bitter and roasted notes. Pyrrols also have been identified in other heated foods such as roasted peanuts, hazelnut, and, coffee, chocolate, cocoa, roasted.26
Beside the tetramethylpyrazine, the most intense peak in the chromatograms was identified as 2-methybutanal and, 3-methylbutanal. They included four Strecker aldehydes, 2-methylpropanal (2), 3-methylbutanal (6), 2-methylbutanal (5), and phenylacetaldehyde (51) respectively derived from Strecker degradation or biodegradation of valine, leucine, isoleucine and phenylalanine. All these degradation processes take place in cocoa beans as a result of the fermentative release of free amino acids.26,31
2-methylpropanal, with strong chocolate notes, most probably have a great impact on the flavor of dark chocolate.26 3-methylbutanal (6) has been identified as one principal responsible for the characteristics chocolate flavor according,32 especially when associated with dimethyldisulfide (11) cited by De Oliveira et al.33 The other detected aldehyde, benzaldehyde (45), already has been identified as odorants in chocolate,26 as well as in other food flavors cited by De Oliveira.33
Frequency distribution of the most relevant volatile compounds of the Criollo cocoa population
Table 3 resume the variability of the main volatile compounds in volatiletic fraction. They were 2-methylbutanal, 3-methylbutanal, 2, 3, 5, 6-tetramethylpirazine, 2-heptanol, and acid acetic.
Peak |
Relevant Individual Volatile Compounds |
Mean %) |
SD |
Inferior Valeur (%) |
Superior Valeur (%) |
5 |
2-methylbutanal |
0.50 |
0.24 |
0.02 |
1.24 |
6 |
3-methylbutanal |
0.50 |
0.31 |
0,004 |
1.28 |
42 |
2,3,5,6-tetramethylpyrazine |
0.50 |
0.41 |
0,00 |
2.02 |
40 |
Acetic acid |
0.50 |
0.32 |
0,00 |
1.52 |
28 |
2-heptanol |
0.50 |
0.35 |
0,02 |
1.60 |
Table 3 Descriptive statistics of the most relevant volatile compounds.
The 2-methylbutanal and 3- methylbutanal are the aldehyde of important contribution to the cocoa volatile.32 They are Strecker-Maillard reaction products, from condentation reaction between sugars and free amino acids; such as valine (2-methylpropanal), leucine (3-methylbutanal), isoleucine (2-methylbutanal), phenylalanine (phenylacetaldehide) and methionine (3-(methyl-tio)-propionaldehyde). The 3-and 2-methylbutanal, and 2-methylpropanal are the compounds that produce a fruit volatiletic flavor and sugary taste typical of the Criollo cocoa.34,35 On the other hand, 3-methylbutanal and phenylethanal have identified as volatile compound of Trinitario cocoa contributing to the typical flavor in milk chocolate and in the honey flavor in the cocoa liquor and powder.26
The contribution of the 3-methylbutanal (or 2-methylbutanal) on the selected cocoa population is following a distribution normal Gaussian (Figure 7A) varying from 0.05 to 1.30% with a mean of 0.60. 3 - methyl butanal represents an important constituent in the " flavor" cocoa and represented a 99% concentration of this compound in the tree population studied
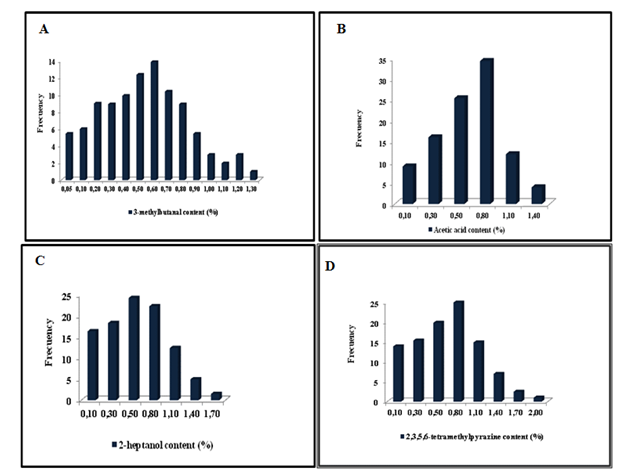
Cocoa type "Criollo" acidity is linked to the fermentation process whose components are usually, acetic acid and 3-methylbutanoic acid. The 98% of the frequency distribution corresponded to the majority of selected materials, ranging from 0.10 to 1.40 (Figure 7B). The acetic acid is a compound produced by the fermentation of the cocoa, involved stage where aerobic bacteria which oxidize ethanol produced by the yeast, acetic acid.10
The alcohols are mostly products of microbiological actions occurring during the fermentation. The 99 % of the frequency distribution for the content of 2-heptanol (Figure 7C) ranged from 0.10 to 1.70% with average of 0.50. Although the overall content of the alcohol has a very low contribution to the volatile of chocolate, certain high concentrations of specific compounds can provide very sharp volatile to the global flavor. For its strong concentration, some alcohols, for example: 2-phenylethanol is associated with typical "floral" volatiles of dark chocolate extracts.
Finally, pyrazines compounds represent the compounds with major importance. They are intensely developed after roasting and are derived from Maillard reactions. Its concentrations of vary with the temperature and time of the roasting treatment (Figure 7D).
Jeanjean,36 Chanliau35 research have shown that concentrations of pyrazines increase rapidly during the post-harvest (fermentation and dry) treatment, they continue to evolve during roasting with the exception of the 2,3- dimethylpyrazine, which is developed during the sun drying. The roasted cocoa mass has a predominant composition, that it is linked to the content of pyrazines which contribute to the flavor, being the tri and tetramethylpyrazine responsible for the strong volatile to "chocolate". Ziegleder,23 Cros et al.,34 Hashim & Chaveron37 have concluded that the increase in the content of methylpyrazines are proportional to the increments of roasting temperature; while 2, 3, 5, 6- tetramethylpyrazine, was slight affected by roasting level. The concentration of 2, 3, 5, 6-tetramethylpyrazine (Figure 8D) follows a normal distribution whose content ranged from 0.10 to 2.00%, and represented 98% of all materials tested. The average distribution was 0.80 % of whole population.
SPME has been used as an alternative analytical tool to measure volatile compounds in the cocoa volatile fraction. The volatile compounds responsible of the volatile of cocoa and chocolate, were analyzed using an analytical method without solvent that has the advantage of being a relatively quick, one-step method of extracting volatile compounds from the headspace, and does not require costly equipment. By using this method, 69 volatile compounds that were grouped into 12 chemical families, were identified. They were grouped in aldehydes, alcohols, acids, hydrocarbons, pyrazines and diverse compounds. All of them are the compounds prevalent in the volatile fraction of roasted cocoa. There is effect of post-harvest treatment (fermentation, drying) and roasted on the formation of flavor compounds in the fraction of roasted cacao. The results of these studies also are show that SPME has a significant advantage over more traditional methods for analysis of volatile compounds in the cocoa beans post-harvest process: no fermented, fermented, dried and roasted. It must to continue these studies in order to characterize elite materials of commercial importance for the country and contribute to generating an appellation of origin cocoa.
None.
Author declares that there is no conflict of interest.

©2016 Álvarez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.