Journal of
eISSN: 2373-4310


Review Article Volume 1 Issue 6
Department of Health, Human Performance and Recreation, Baylor University, USA
Correspondence: Darryn Willoughby, Department of Health, Human Performance, and Recreation, Baylor University, Waco, 1312 S. 5th Street, P.O. Box 97313, Waco, TX 76798-7313, USA, Tel 254-710-3504
Received: November 01, 2014 | Published: November 28, 2014
Citation: McKinley S, Willoughby DS. Effectiveness of antioxidant nutraceuticals in attenuating canonical NF- κb signaling in human skeletal muscle resulting from exercise-induced inflammation and oxidative stress. J Nutr Health Food Eng. 2014;1(6):214-221. DOI: 10.15406/jnhfe.2014.01.00033
Oxidative stress is a process that produces cellular damage by oxidation of cellular components such as lipids, protein, and DNA. It is also a regulator of the acute phase inflammatory response, and also involves the production of reactive oxygen species (ROS) by mitochondrial respiratory activity and from the capillary endothelium in contracting skeletal muscles. Exercise-induced muscle damage is associated with an acute phase inflammatory response. This response results in the production of circulating pro-inflammatory cytokines such as tumor necrosis alpha (TNF-α) which is a mediator of muscle wasting and acts through the stimulation of nuclear factor kappa B (NF-κB). Skeletal muscle damage and injury becomes evident in many catabolic scenarios and is characterized by proteolysis and muscle wasting. Inflammation and oxidative stress are associated with skeletal muscle wasting in various disease states including cancer cachexia, muscular dystrophy, AIDS, sepsis, etc. Antioxidant supplementation has been suggested to attenuate exercise-induced oxidative stress and the subsequent activation of NF-κB through various mechanisms; however, there is still conflicting evidence and more research needs to be completed to better understand the role of NF-κB signaling in skeletal muscle. Antioxidant supplementation has also been shown to attenuate oxidative stress and the subsequent formation of reactive oxygen nitrogen species (ROS, RONS) in skeletal muscle. As a result, this area requires further research as this process has also been demonstrated to mediate NF-κB signaling. Prior evidence suggests that various nutraceutical supplements seem to play a beneficial role in maintaining muscle mass during periods of heightened inflammation and oxidative stress and possible muscle wasting.
Keywords: oxidative stress, inflammation, skeletal muscle, proteolysis, cytokine, antioxidant, nutraceutical
ROS, reactive oxygen species; RONS, reactive oxygen nitrogen species; TNF, tumor necrosis factor; TNFR, tumor necrosis factor receptor; NF, nuclear factor; COPD, chronic obstructive pulmonary disease; RHD, rel homology domain; IL, interleukin; NIK, NF-κB inducing kinase; IKK, IκB kinase; BCL, b-cell lymphoma; MOD, minimal oligomerization domain; LPS, lipopolysaccharide; RIP, receptor interacting protein; TRAF, tnf-receptor associated factor; TRADD, tnfr-associated death domain protein; c-IAP, cellular inhibitor of apoptosis protein; TAK1, tgf-β-activated kinase 1; LT-β, lymphotoxin β; BAFF, b-cell activating factor; CD, cluster of differentiating; MuRF1, murine ring finger-1; UPS, ubiquitin proteasome system; TWEAK, thf-like weak inducer of apoptosis; HSPs, heat shock proteins; NFAT, nuclear t factor of activated t cells
Exercise preferentially biased with forced-lengthening (eccentric contractions) causes muscle damage, which is a manifestation of ultra-structural changes and protein degradation, and is associated with the development of an acute phase inflammatory response.1 This inflammatory response is a microvasculature-induced reaction characterized by the migration of serum proteins and leukocytes from the blood to the skeletal muscle. Involved in this migration are pro- inflammatory cytokines, most notably tumor necrosis factor alpha (TNF-α)2 which binds its trans-membrane receptor in skeletal muscle and activates a transcription factor known as nuclear factor kappa B (NF-κB). The activation of NF-κB has been found to be a critical factor involved in the multifaceted homeostasis of muscle. This becomes important as the development and growth of skeletal muscle requires a complicated series of events that are regulated through various pathways. The plasticity of skeletal muscle is important to regulate the need for anabolic and catabolic processes, and any perturbation of these processes can lead to various muscle disorders associated with wasting.3
Furthermore, exercise-induced muscle damage is also associated with oxidative stress, a process that not only directly causes cellular damage by oxidation of cellular components such as lipids, protein, and DNA, but also acts as a regulator of the acute phase inflammatory response. Oxidative stress instigates the nuclear translocation of certain redox-sensitive transcription factors, pro-inflammatory cytokines, chemokines, and adhesion molecules. The resultant effect is phagocyte infiltration into skeletal muscle, thereby causing proteolysis, ultra-structural damage, and oxidative injury. During this cascade is the production of reactive oxygen species (ROS) by mitochondrial respiratory activity. In addition, due to the intermittent ischemia-reperfusion associated with exercise, xanthine oxidase is activated resulting in the formation of ROS by the capillary endothelium in contracting muscles.4
Skeletal muscle damage and injury becomes evident in many catabolic scenarios and is characterized by proteolysis and muscle wasting. Inflammation and oxidative stress are associated with skeletal muscle wasting in various disease states including cancer cachexia, muscular dystrophy, AIDS, sepsis, diabetes, chronic obstructive pulmonary disease (COPD), and cystic fibrosis.5‒9 Muscle wasting occurs as a result of a reduction in protein synthesis, an increase in protein degradation, or both.10‒12 Various stimuli prompt skeletal muscle wasting through specific mechanisms and exercise-induced muscle damage is a fitting model for this process. While multiple signaling pathways are involved in the process of skeletal muscle wasting, recent evidence has suggested that NF-κB is one of the most important mechanisms because the activation of the NF-κB pathway stimulates muscle loss.5 The pro-inflammatory cytokine, TNF-α, is a mediator of muscle wasting and acts through the stimulation of NF-κB.5,13 Recent evidence has shown that modification of NF-κB activity through pharmacogenetics can actually prevent skeletal muscle loss in various diseases, injury, and unloading.14‒17
There is not an abundance of human studies demonstrating the role that antioxidant supplements may play in down-regulating NF-κB activity. However, enough evidence does exist suggesting a potential beneficial role for this type of nutraceutical supplementation. Therefore, it is feasible to review the potential for various antioxidant nutraceuticals on NF-κB activity, and the subsequent capacity they might possess in attenuating the effects of catabolic NF-κB activity, on oxidative stress and wasting in skeletal muscle.18‒21 Therefore, the purpose of this brief review is to better understand the underlying mechanisms of NF-κB and its effects on oxidative stress and muscle wasting in humans, as well as possible antioxidant nutraceutical supplements that may attenuate these effects.
NF-κB is a ubiquitously expressed transcription factor that is considered vital to numerous cellular processes.3,22 It has been suggested that NF-κB directly modifies hundreds of gene products, including genes that encode cytokines, chemokines, cell adhesion molecules, growth factors, immunoregulatory receptors, acute-phase and stress response proteins, cell surface receptors, transcription factors, and several enzymes involved in protein degradation by the ubiquitin-proteasome system, as well as regulators of redox status, apoptosis, disuse atrophy, and host defense.5,23 This is summarized in Figure 1. The NF-κB family is comprised of five genes that code for protein subunits, RelA/p65, RelB, c-Rel, p50, and p52, which can be seen in Figure 2A.3,5,13,22 In humans, the proto-oncogene c-Rel is a protein that is encoded by the REL gene (v-rel avian reticuloendotheliosis viral oncogene homolog). The c-Rel protein is a member of the NF-κB family of transcription factors and contains a Rel homology domain (RHD) at its N-terminus and two C-terminal trans activation domains. As can be seen in Figure 2B, the IκB family consists of seven proteins including IκBα, IκBβ, IκBε, IκBγ, B-cell lymphoma 3-encoded protein (BCL-3), and precursor proteins p100 and p105.5,13,22,24 The most commonly described forms of NF-κB are the p50/p65 heterodimer and p50/p50 homodimer complexes, followed by p50/c-Rel and p52/RelB.3,10 Unlike the other genes, p50 and p52 genes lack transcriptional activation domains, which generates p50 and p52 homodimers that function as gene repressors by blocking DNA consensus sites, which can be seen in Figure 3.3,5,7,22
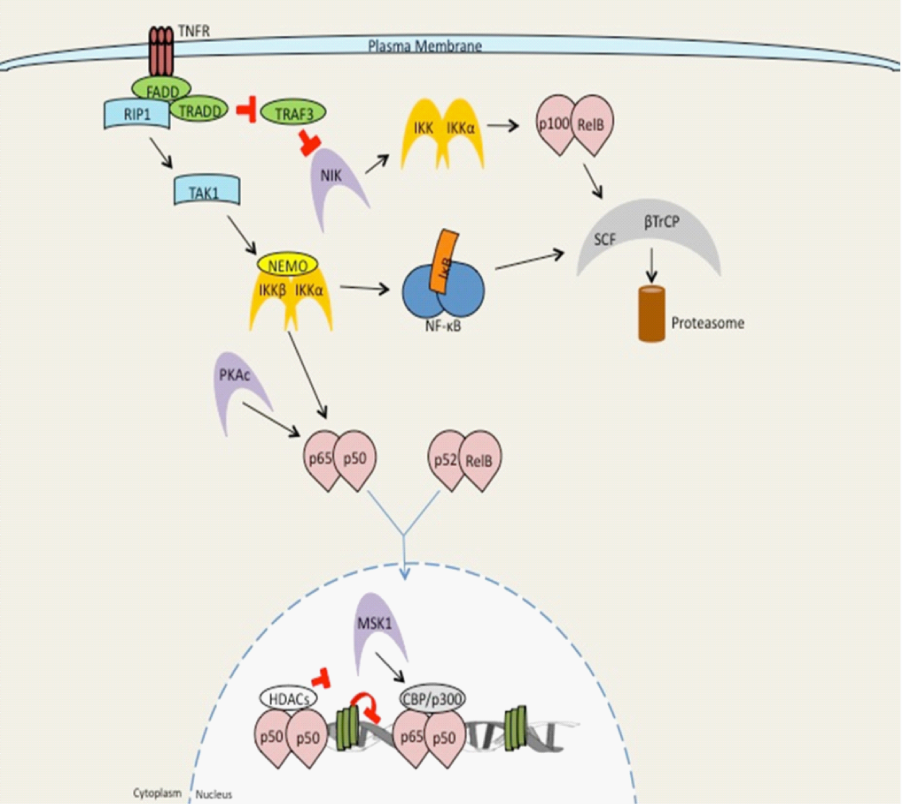
Figure 1 Overview of NF-κB signaling by TNF receptor signaling.
Signaling by members of the TNF receptor super family, such as TNFR1, leads to recruitment of the adaptor proteins FADD and TRADD, TRAF family members (TRAF2, 5, and 6) and the kinase RIP1. The TRAF/RIP complex recruits and activates TAK1, which induces activation of the IKK complex and subsequent downstream NF-κB signaling. Upon stimulation, a subset of TNFR super family members that bind to TRAF3 (CD40, LTβR, BAFFR) induce TRAF3degradation resulting in accumulation of the kinase NIK. NIK then undergoes constitutive degradation in the absence of stimulation. Accumulated NIK phosphorylates and then activates IKKα. IKKα, thereby inducing processing of the NF-κB family member p100 into p52. At this point p52-containing NF-κB complexes become activated (prototypically RelB: p52). Modified from Hayden et al. [57].
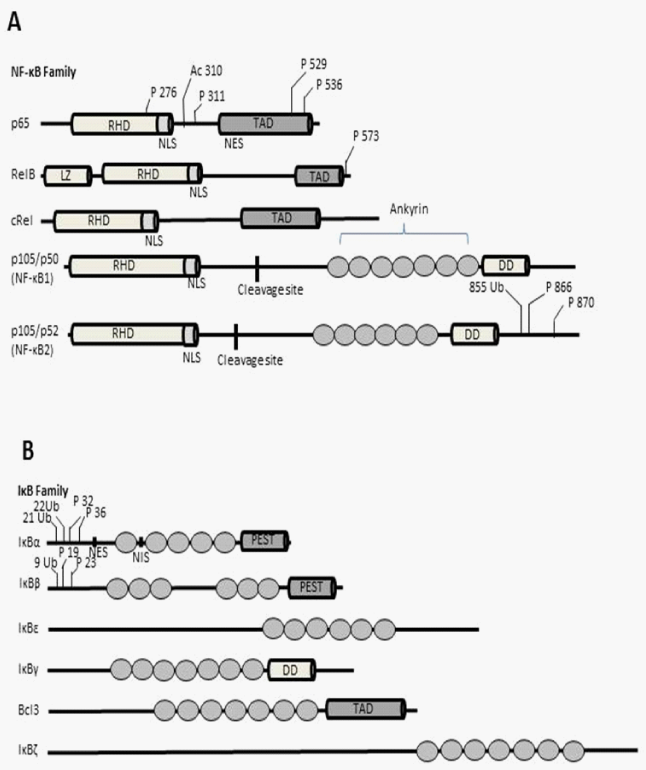
Figure 2 In A: human NF-κB protein and in B: IκB proteins are illustrated. In A and B, the specific post-translational modifications of both are also illustrated.
P: Phosphorylation; Ac: Acetylation; Ub: ubiquination; TAD: Trans Activation Domain; RHD: Rel Homology Domain; NES: Nuclear Export Signal; NLS: Nuclear Localization Signal; NIS: Nuclear Import Signal; LZ: Leucine Zipper; DD: Death Domain; Ankyrin, ankyrin repeats; PEST: Polypeptide Sequences Enriched in Proline (P); glutamate (E); serine (S) and threonine (T) [22].
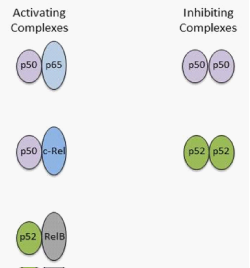
Figure 3 The NF-κB family members represented by the activating and repressor NF-κB heterodimers.
In regard to the activating complexes, these include the p50/p65, p50/c-Rel, and p52/Rel B heterodimers. Relative to the inhibiting complexes, these involve the p50/p50 and p52/p52 homodimer complexes. Contrary to the other genes, the p50 and p52 genes lack transcriptional activation domains, which generates p50 and p52 homodimers, and function as gene repressors by blocking DNA consensus sites [3].
Prior to activation, NF-κB dimers are held in the sarcoplasm bound to inhibitory proteins, termed inhibitor of NF-κB (IκB).3,5,13 While NF-κB is bound to IκB, nuclear translocation is averted, which maintains the inactive state of NF-κB in the cytoplasm.5 However, when IκB proteins are degraded following stimulation of the cell, nuclear entry of NF-κB dimers becomes favoured.22 Specifically, once the cell is stimulated, the IκBα protein is quickly phosphorylated at serine 32 and 36, which activates poly-ubiquination and degraded by the 26S proteasome, thereby allowing nuclear entry of NF-κB dimers.13 NF-κB dimers are then free to travel to the nucleus where they bind to κB sites within target gene promoters. NF-κB, along with other transcription factors, then regulates transcription of genes (Figure 4).24
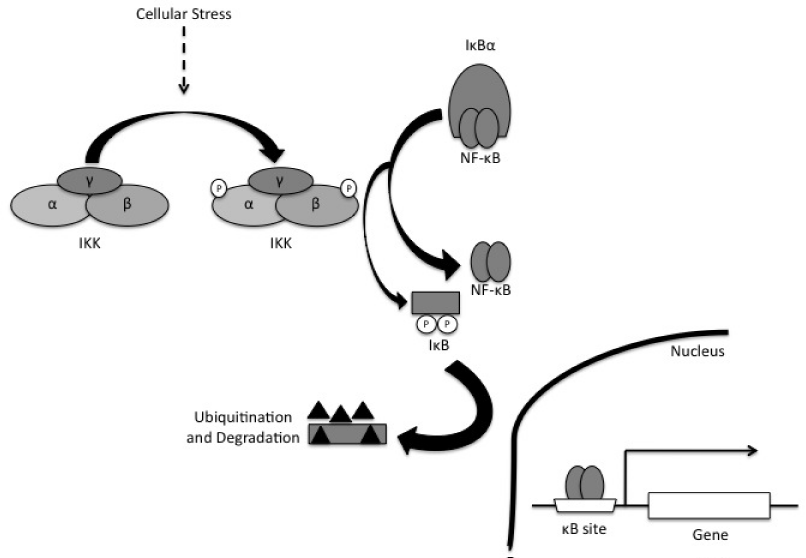
Figure 4 Activation of IκB/NF-κB pathway by a physiological induction with stimuli such as exercise. The contraction of skeletal muscle results in transient increases in cell stress, as evidenced by the accumulation of ROS, energy depletion, etc. that induces phosphorylation of IκBα kinase (IKK) β. The activated IKKβ phosphorylates the inhibitory peptide IκBα, thereby activating it for ubiquination and degradation by the proteasome. At this point, the freed NF-κB translocates to the nucleus and forms dimers to bind DNA and induce trans activation.13
Activation of the NF-κB complex occurs in response to various stimuli, including infection, exposure to pro-inflammatory cytokines, mitogens, growth factors, biomechanical stressors, and oxidative stressors. There are several pathways by which NF-κB can be activated following ligation of different receptor families, including tumor-necrosis factor receptor (TNFR) and interleukin-1 receptor (IL-1R). NF-κB complex activation also includes the activation of several intermediate kinases, including NF-κB inducing kinase (NIK). These intermediate kinases act as distinct signaling proteins that all unite on the IκB kinase (IKK) complex.5,24
The IKK complex controls the breakdown of IκB proteins through regulation of the phosphorylation of serine 32 and 36 on IκBα, and serine 19 and 23 on IκBβ. This phosphorylation results in K48-linked polyubiquitination by the SCFβTrCPE3 ubiquitin ligase complex on lysine 21 and 22 of IκBα, which is an ATP-dependent occurrence that quickly targets the breakdown of these proteins by the 26S proteasome.3,22 To fully understand the NF-κB pathway, it is necessary to understand the IKK complex and its role in NF-κB activation.
IKK Complex
The IKK complex is composed of two catalytic subunits IKKα/IKK1 and IKKβ/IKK2, and one non-catalytic regulatory subunit IKKΥ/NEMO (NF-κB essential modulator).3,5,22,24,25 IKKα and IKKβ maintain a strong sequence homology, especially in their catalytic region, but differ in their specific targets.3,22,24 Both kinases enhance NF-κB trans activation potential by phosphorylation of p65 at serine 536. However, IKKβ can further enhance NF-κB activity by targeting serine 468 of p65. Conversely, IKKα phosphorylates p100 to stimulate partial proteolysis and activation of the alternative NF-κB pathway.3 NEMO interacts with both IKKα and IKKβ, and associates with intermediates of upstream signaling.24
IKK complex activation requires phosphorylation of either IKKα or IKKβ on two particular serine residues within the T loop of the catalytic domain of each kinase. Unfortunately, the kinase(s) that are responsible for T loop phosphorylation are not known. There are two possible mechanisms that have been suggested that may be responsible for phosphorylation. The first potential mechanism is that the IKK proteins phosphorylate each other by trans-autophosphorylation, which is facilitated by either conformational changes within the IKK complex or induced proximity through oligomerization of multiple core IKK complexes. The second possible mechanism could be the existence of separate IKK-kinases that target these residues with conformational changes within the complex exposing the T loop for phosphorylation. It may even be possible that both mechanisms may happen independently or together in a nature dependent manner of the particular upstream signaling pathway (Figure 5).24
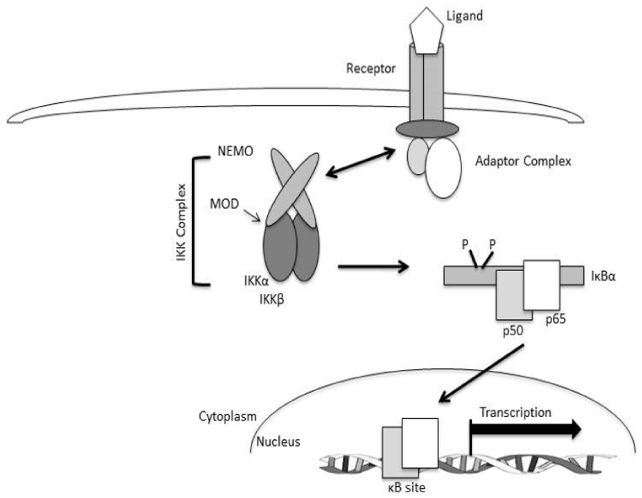
Figure 5 The NF-κB signaling pathway which depicts the major components of canonical signaling. The engagement of the ligand (TNF-α) binding to its receptor (TNFR) induces the formation of an adaptor-protein complex containing adaptor and scaffolding proteins such as TRAFs, MyD88, and TRAP, as well as kinases such as RIP and IRAK). The assembly of these protein complexes facilitates the recruitment and activation of the IKK complex through NEMO which, in turn, leads to phosphorylation and ultimately ubiquination and proteasomal degradation of IκB proteins. IκB degradation reveals a nuclear localization sequence on the p50-p65 heterodimer allowing them to migrate to the nucleus, bind to specific κB-promoter sites and regulate target gene transcription [24].
Unlike the mechanisms responsible for T loop phosphorylation, the role of NEMO-induced activation of IKK has been well established. It has been determined that cells absent of NEMO are unable to activate NF-κB in response to most pro-inflammatory, immunoregulatory, and pro-survival stimuli.26‒28 NEMO associates with IKKα and IKKβ as a dimer, and can also oligomerize by a minimal oligomerization domain (MOD). Following NEMO stimulation, induced oligomerization seems to be critical for activation of IKK, and any mutations within the MOD blocks activation of IKK.24 Recently, it has been suggested that NEMO is not only an activator of IKK complex, but may also play a role in the control of IKK down-regulation through a negative interaction with IKKβ.29‒31 However, the mechanism(s) by which this occurs is not known. NEMO-induced activation of IKK, and recently the role of NEMO in repression of IKK is still the focus of ongoing research, and knowledge about NEMO-induced IKK activation and repression is continuing to develop.
NF-κB signaling pathways
Depending on which subunit of the IKK complex is involved, activation of NF-κB can occur through one of two pathways termed the classical/canonical and alternative/non-canonical pathways.3,5,22,24,25 Recent evidence has shown that both pathways can be activated in skeletal muscle, with either of these pathways capable of causing skeletal muscle wasting.15,16,32 The classical pathway is IKKβ- and IKKγ-dependent, with NF-κB activation occurring due to the degradation of IκB proteins. This pathway is activated in response to inflammatory factors, bacteria, oxidative stress, leukocyte adhesion molecules, and various pro-survival genes.3,22,24 Activators of this pathway include TNF-α, interleukin 1 (IL-1), and lipopolysaccharide (LPS), which triggers a receptor-mediated assembly of a multi-protein signaling complex, due to interaction with receptor interacting protein (RIP), that leads to activation of IKK. As a member of the TNF-receptor associated factor (TRAF) family, TRAF1-7, is at the forefront of this pathway and acts as a E3 ubiquitin ligase. TNF-α binds to its receptor, appropriately termed TNFR, which causes TNFR-associated death domain protein (TRADD), TRAF2, and receptor-interacting protein kinase (RIP) to be recruited. NF-κB activation by TRAF2, in association with cellular inhibitor of apoptosis protein (c-IAP) induces K-63 linked poly-ubiquitination of RIP, which activates TGF-β-activated kinase1 (TAK1). TAK1 then directly phosphorylates IKK, which results in the degradation of IκB proteins, and subsequent activation of the classical p50/p65 complex.3
NF-κB can also be activated through its alternative pathway by lymphotoxin β (LT-β), B-cell activating factor (BAFF), and cluster of differentiating 40 (CD40). Conversely to the classical pathway, the alternative pathway relies on IKKα and NF-κB-inducing kinase (NIK), as opposed to IKKβ and RIP.3,5 NIK phosphorylates IKKα, which results in activation and phosphorylation of IκB protein p100 on sites serine 866 and 870. Unlike other IκB members, p100 undergoes only partial proteolysis, resulting in a functional p52 subunit. This functional p52 subunit is then released and transported to the nucleus in complex with RelB. Figure 6 depicts the two pathways of NF-κB.
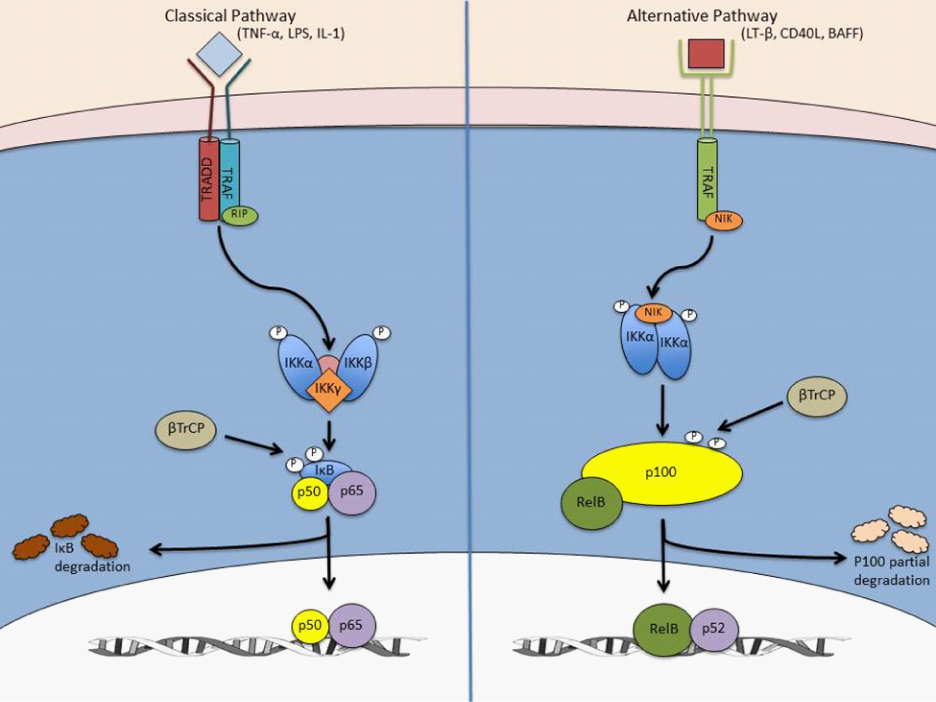
Figure 6 lassical and alternative pathways of NF-κB signaling pathways.
The classical, canonical, signaling pathway is initiated by the binding of either TNF-α, IL1, or LPS to its receptor and the subsequent sequential recruitment of the adaptors TRADD, RIP, and TRAF to the sarcolemma. IKK complex assembly and recruitment to the sarcolemma occurs between IKKα, IKKβ, and IKKγ, thereby resulting in IKKβ phosphorylation and activation. IKKβ then phosphorylates IκBα to promote polyubiquination and subsequent immediate proteasomal degradation through β-TrCP. In contrast, the alternative, non-canonical, signaling pathway, the p65/p50 heterodimer is free to translocate to the nuclease and mediate transcription of NFκB target genes, including one of its own inhibitor IκBα. Conversely, alternative NFκB signaling is activated by ligands, CD40L, and lymphotoxin β that mediates recruitment of TRAFs to the membrane-bound receptor and subsequently activates NIK, thereby phosphorylating IKKα homodimer. Upon activation, IKKα phosphorylates p100 which instigates partial proteolysis by β-TrCP to produce the p52 subunit. As a result, the RelB/p52 complex translocates to the nucleus to transcribe respective target genes [22].
It has been determined that murine C2C12 skeletal muscle cell lines contain p65/p50 in their nucleus, which is the most commonly studied form of NF-κB. In the myoblast cell line C2C12, NF-κB binds on κB sites of the cyclin D1 promoter. This then allows for transcription regulation, leading into the S phase of the cell cycle. During myogenesis, binding activity of NF-κB on cyclin D1 is decreased. This suggests that NF-κB is a critical element regulating transition to the differentiation stage from the proliferation phase. During atrophic conditions, NF-κB binds on the promoter of murine ring-finger-1 (MuRF1), which is an E3 ubiquitin ligase.25 This increases expression of MuRF1, suggesting NF-κB regulates the ubiquitin-proteasome system (UPS), resulting in muscle wasting.3,25
Another potential mechanism by which increased NF-κB activity leads to skeletal muscle wasting is the possibility of NF-κB increasing the expression of inflammation-related molecules, which either directly or indirectly enhances muscle wasting. Pro-inflammatory cytokines, including TNF-α, THF-like weak inducer of apoptosis (TWEAK), IL-1, and IL-6 are major inducers of muscle wasting. Evidence has shown that NF-κB regulates expression of cytokines, chemokines, and cell-adhesion molecules.33 It has also been determined that NF-κB inhibition promotes regeneration of skeletal muscle by limiting the inflammatory response.17
Various physiological stressors such as disease and exercise, which causes muscle injury, are known to induce oxidative stress. After injury, infiltrating leukocytes exert antiseptic protection of muscle by releasing reactive oxygen species (ROS) through oxidative burst activation of NADPH oxidase.34 This process results in marked perturbations of redox status in muscle fibers.35 Consequently, pro-inflammatory cytokines, including TNF-α, are released by neutrophils and injured muscle fibers, thereby activating ROS-generating enzymes such as xanthine oxidase.36 When ROS production is greater than the capacity of the antioxidant defense system, an oxidative stress occurs resulting in many cellular contents suffering oxidation due to ROS attack37 and subsequent activation of NF-κB, activator protein 1 (AP-1), and forkhead box (FOXO) proteins. Additionally, increased levels of ROS results in oxidation of several proteins in the excitation-contraction mechanism,38 and the production of protein reactive carbonyl derivatives lead to a loss of catalytic activity and increased vulnerability to protein breakdown.39 The effects of ROS production on signaling pathways in skeletal muscle can be seen in Figure 7.

Figure 7 Effects of ROS production on skeletal muscle signaling pathways. The release of ROS from mitochondria, induced by humoral mediators such as NF-κB, activator protein 1 (AP-1), and forkhead box (FOXO) proteins, or metabolic alterations results in the activation of various intracellular signaling pathways such as the proteasome system and mitogen activated protein kinase (MAPK)that collaboratively work to increase both proteolysis and cytotoxicity in the skeletal muscle [19].
It is well known that cellular stress is an activator of the NF-κB classical pathway, specifically ROS formation. Sen et al.,40 were the first to demonstrate that TNF-α-induced activation of NF-κB in L6 myocytes, which was augmented by conditions of oxidative stress. It was also determined that inhibition of NF-κB activity by the antioxidant pyrrolydine dithiocarbamate, coupled with an increase in ICAM-1 expression, suggested that the intracellular redox status was critical in activation of NF-κB, nuclear translocation, and subsequent gene transcription regulation of proteolytic genes.41 Li et al.,6 also found that TNF-α led to ROS-mediated NF-κB activation, resulting in a decrease of total protein in skeletal muscle with a specific loss of myosin heavy chain. The enhanced muscle protein degradation was coupled with TNF-α-dependent stimulation of total ubiquitin conjugation of myotube proteins.6
Exercise causes mechanical and metabolic stresses on skeletal muscle, including an oxidative and inflammatory response.3 Various cell models have shown that increased ROS accumulation activates NF-κB.42,43 This has led to the hypothesis that exercise may also activate NF-κB.13 This hypothesis has gained support because several studies44‒48 have shown that, in addition to calcineurin-nuclear T factor of activated T cells (NFAT) signaling and heat shock proteins (HSPs), NF-κB can be also be activated with exercise. Ji et al.,46 determined that rats exercised to exhaustion had increased levels of NF-κB activation when compared to control. Ho et al.,44 also reported an increase of NF-κB activity by 50% 1-3 three hours following a 60 minute treadmill running bout. As much as a 76% decrease in IKK phosphorylation was also seen. Another mechanism suggested due to exercise is that acute exercise may cause oxidation of glutathione in skeletal muscle, which results in increased oxidative stress and NF-κB activation.49 Evidence from this example suggests that regular exercise may increase glutathione levels in skeletal muscle, resulting in a down-regulation of NF-κB activity.41 However, there have been a few studies that have shown that exercise causes a down-regulation of NF-κB activity.32,50,51 While the majority of evidence favors exercise-induced NF-κB activity, more research needs to be completed to determine why there are some discrepancies in the research. The relationship between exercise, ROS, and RONS (reactive oxygen nitrogen species) can be seen in Figure 8.
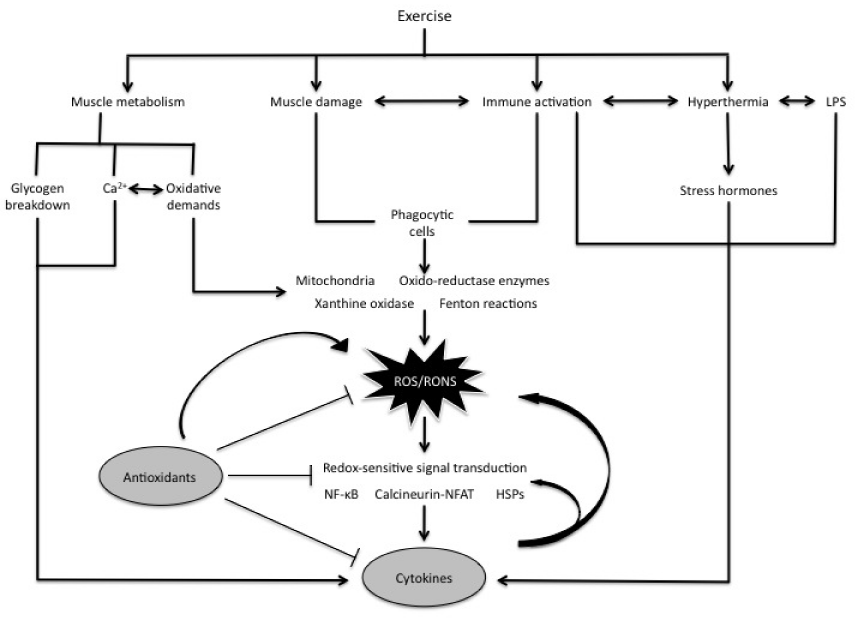
Figure 8 Interactions between exercise, ROS/RONS, antioxidants, and cytokines. Exercise causes mechanical and metabolic stresses on skeletal muscle, thereby inducing an oxidative and inflammatory response. As a result, ROS accumulation occurs and activates NF-κB, in addition to calcineurin-nuclear T factor of activated T cells (NFAT) signaling and heat shock proteins (HSPs) [20].
Antioxidant supplementation has been suggested to attenuate exercise-induced oxidative stress. Redox regulation of cytokine production occurs at multiple levels, including the activation of oxidant-sensitive and redox-sensitive NF-κB.52 Anti-oxidant supplementation has been show to attenuate NF-κB, activated by ROS associated with exercise-induced muscle damage53 and RONS.54 Aoi et al.,53 demonstrated in rats that a 3-week diet high in α-tocopherol (500mg/kg) attenuated NF-κB activation and p65 nuclear translocation following treadmill running for 60min at a speed of 25m/min. This study showed there is a relationship between ROS and NF-κB and that supplementation of the antioxidant α-tocopherol was able to inhibit oxidative stress and NF-κB activation. Vassilakopoulas et al.,55 investigated the effect of a multiple antioxidant supplement (vitamins E, A, C for 60days and N-acetyl cysteine for 3days) in healthy males on plasma cytokine concentrations following bicycle exercise for 45minutes at 70% of maximum oxygen consumption. They determined, compared to pre-supplementation, that anti-oxidant supplementation abolished the exercise-induced increase in circulating TNF-α. Michailidis et al.,56 showed that the thiol-based antioxidant N-acetyl cysteine provided orally to men at a dose of 200mg/kg/day for 8days effectively attenuated circulating pro-inflammatory cytokines and skeletal muscle NF-κB phosphorylation following muscle damaging exercise (300 eccentric contractions).
The information presented herein indicates that various antioxidant nutraceuticals can play a beneficial role in attenuating oxidative stress and the subsequent increase in circulating TNF-α and canonical NF-κB signaling occurring in response to exercise-induced muscle damage. Since the downstream consequences of exercise-muscle damage are similar to those associated with various pathologic conditions involving muscle wasting, the implications from this review are noteworthy. Inhibition of NF-κB pathway activity has been shown to attenuate muscle wasting through various mechanisms; however, there is still conflicting evidence and more research needs to be completed to fully elucidate the role of NF-κB signaling in skeletal muscle. Antioxidant supplementation has been shown to attenuate oxidative stress and the subsequent formation of ROS/RONS production in skeletal muscle. Furthermore, this area requires further research as this process has also been demonstrated to mediate NF-κB signaling. Research on NF-κB signaling in skeletal muscle is a rapidly growing area of interest, and advances in this area may affect the types of nutraceutical supplements ingested with the intent of maintaining muscle mass during periods of heightened inflammation and oxidative stress and possible muscle wasting.
This brief review project was not partially or fully sponsored by any external sponsoring agent, rather support was provided by the Department of Health, Human Performance, and Recreation of Baylor University.
Author declares that there is no conflict of interest.

©2014 McKinley, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.