Journal of
eISSN: 2373-4310


Research Article Volume 5 Issue 2
Program in Nutrition and Complementary Medicine, University of Science, Arts and Technology, USA
Correspondence: Tony Brown, Program in Nutrition and Complementary Medicine, College of Medicine, University of Science, Arts and Technology, Montserrat, British West Indies, USA, Tel 727-388-2687; 664-491-5364, Fax 664-491-5362
Received: December 31, 2014 | Published: November 7, 2016
Citation: Tulp OL, Brown T. Effect of a fructan-chromium complex on glycemic responses of congenic obese la/ntul//- cp rats. J Nutr Health Food Eng. 2016;5(2):594-598. DOI: 10.15406/jnhfe.2016.05.00167
Recent studies have shown that the marked adiposity of the obese phenotype of the LA/Ntul/ /-cp rat to be associated with hyperinsulinemia, hyperamylinemia, and impaired glucose tolerance following an oral glucose challenge when compared to glycemic responses of similarly-fed lean littermates, and to be linked to a hypothalamic receptor defect for the obesity hormone, leptin. To determine the effects of an inulin fructan polymer-chromium complex (FCC) derived from dahlia root juice on glycemic responses, groups of congenic lean and obese LA/Ntul//-cp rats were administered an oral glucose tolerance (OGT) alone or in combination with measured amounts of the FCC. Glycemic responses and the glucose area under the curve (AUC) of obese rats were significantly impaired compared to similarly-fed lean littermates. Oral FCC administration resulted in a significant 50% reduction toward normal of the peak glycemic response during OGT in both lean and obese rats, a 75% reduction in the total glucose area of lean rats and a 50% reduction of the total glucose area of obese rats. The greatest segment of the reduced glucose area occurred during the post absorptive phase of the OGT response in both phenotypes. The results of this study are consistent with an acute FCC-mediated improvement in glycemic control in a congenic obese animal model of impaired glucose tolerance, and suggest that the FCC complex may be a useful nutraceutical adjunct in the dietary treatment of obesity and other glucose-intolerant states as they may occur in man and animals.
Keywords: fructan, inulin, glucose tolerance, obesity, rat, chromium
FCC, fructan polymer-chromium complex; OGT, oral glucose tolerance; AUC, area under the curve; IGT, impaired glucose tolerance; GTF, glucose tolerance factor
Impaired glucose tolerance (IGT) is a common metabolic feature of obesity in man and animals, where it is associated with graded impairments in insulin action in muscle and adipose tissue, and in impaired glycemic control. The IGT responses may be linked in part to deficiencies in micronutrient availability, suboptimal macronutrient ratios, excessive macronutrient and total caloric intake, inadequate ingestion of dietary fiber or other indigestible residues, impairments in macronutrient metabolism, or some combination of these factors. Specific foods have been used by many peoples to control glycemic responses and metabolism and to therefore modulate or ameliorate the progression of typical sequellae which may become linked to exaggerated hyperglycemic responses. Many foods known to contain such nutritional factors have been used in an attempt to modulate glycemic responses, with a general goal of returning the glycemic responses toward normal, with secondary benefits of decreasing hyperglycemic contributions to obesity, diabetes, cardiovascular, and other nutritionally linked disorders. Therapeutic considerations for proper and effective treatment of IGT and its sequellae may include careful integration of a wide range of therapies, including adjustments to both lifestyle and diet. Dietary fibers, including both digestible and indigestible fibers, along with bioavailability of specific micronutrients also contribute to improvements toward normalization of glycemic responses in glucose intolerant states.
Inulin is an indigestible, relatively low molecular weight fructan polymer often found in low glycemic index foods. The D-fructofuranosyl carbohydrate units of insulin form a-[l->4 polymer containing 20-30 fructose residues via glycosidic Iinkages].1 Mammalian luminal digestibility of the polymers is typically limited to a single C-2 linked D-glucopvranosyl unit of the terminal reducing end of the polymer, which gives rise to a terminal sucrose unit, while limited microbial degradation of the remaining polymers may occur in the presence of colonic bifidobacteria species.2#ref In addition, inulin and other oligofructose polymers have also been reported to stimulate a beneficial propagation and metabolic activity of bifidobacteria species and to suppress the propagation of bacteroides, fusobacteria, clostridia species, and other microorganisms that are considered less healthful to the colonic tissues.3 The specific mechanism through which the bifidobacterial species inhibit non-beneficial colonic bacterial species is unknown, but has been proposed to be linked to fermentation-linked acid production, resulting in colonic pH below that which pathogenic bacterial species are able to compete for nutrients and thrive effectively.4
The Fructan polymers are typically most commonly found in roots and tubers of the Compositae family, including such plant species as asters, dandelions, dahlias, cosmos, goldenrod, chicory, Jerusalem artichokes, and others. Additionally, frutans have also been isolated from roots and bulbs of members of the Liliacae family, including the bulbs of onions, lilies, hyacinth, and tulips, as well as from some species of algae.1 Because of their indigestable β-glycosidic linkage in humans and their moderate molecular size (3,000-5,000Da), fructans remain in the gastrointestinal contents throughout much of their passage through the gut, where they impart favorable effects on gastric emptying, luminal viscosity, and absorptive processes for nutrients.5 Because of these physicochemical characteristics, combined with relatively neutral organoleptic properties, fructans are often consumed with functional foods, and as a component of nutritional supplement formulations designed to attenuate the intestinal absorptive characteristics of meals.
Obesity’ occurs in the congenic VAF-SPF LA/Ntul//-cp rat as the result of an autosomal recessive trait originally derived from the Koletsky rat, and transmitted into the LA/N and SHR/N background strains.6 Animals were then subjected to 12cycles of backcrossing to eliminate non-corpulent traits, and to reestablish the congenic status. Incorporation of the -cp trait resulted in early onset hypertrophic-hyperplastic obesity which typically becomes visibly evident by approximately 5 to 6weeks of age in affected offspring.7 The obese phenotype is associated with hyperphagia, marked hyperlipidemia, hyperinsulinemia, hyperamylinemia, hyperleptinemia, and an impaired glucose tolerance similar to that which may occur in obese humans,8‒11 and an impaired thermic response to alterations in diet and environment,7,8,12 Because of the homogeniety conferred by the congenic and SPF-VAF status of this strain, however, characteristics of growth, metabolism, aging, and the metabolic responses to diet and environment of different animals are more similar in this rodent population within phenotypes than might be observed in a less homogenous model or in free-living human subjects.7
The purpose of the present study was to determine the effects of an inulin-chromium complex, consisting of an indigestible β-linked glycan of fructan units, on glycemic responses of congenic obese rats. This complex is the major ingredient of DahluleanTM, a dietary supplement used as a nutraceutical adjunct intended as an appetite modulator for the dietary treatment of glucose intolerant states associated with obesity, NIDDM, and overweight conditions. While individual responses to FCC administration in humans are likely to vary somewhat, however, because of the genetic, environmental, and metabolic diversity inherent in a free living human population, individual variation of metabolic responses within a congenic animal model such as the one selected are likely to be minimized because of the genetic and environmental homogeniety of the test population. The purposes of the present study therefore were to quantify the specific effects of single-dose administration of FCC on glycemic responses in a congenic animal model highly predisposed to obesity, glucose intolerance, and insulin resistance, so that the specific glycemic responses could be more accurately assessed than in non-congenic animal populations or human populations.
Groups of lean and obese male LA/Ntul//-cp rats were obtained from the Drexel University Colony as biological littermates at 3months of age and randomly assigned to the study (n=6 rats/group) Animals were fed Purina Rodent chow.#5400 and house water, ad libitum, and maintained in a biobubble with standard lighting (d2000-0800h) and 50% relative humidity throughout the study. Body weights of all rats were obtained periodically with an electronic animal balance.7 Rats were subjected to studies of a 4-point oral glucose tolerance (OGT; 2.5 g glucose/kg BW, p.o. via intragastric gavage as a 50% w/w glucose solution)11 after a brief (4-6hours) fast in the forenoon hours (1000-1200 hr), or subjected to the OGT in the presence of the fructan-chromium complex (FCC: 2.5g/kg BW, p.o. via intragastric gavage), or both glucose and FCC sequentially over a 5minute period. Bloods were obtained via tail bleeding before administration of the glucose or FCC, and again after 30, 60, and 120minutes following the oral glucose or FCC challenge. Blood glucose concentrations were measured in tail blood with use of a rapid glucose oxidase method (Glucometer EliteTM, Ames Laboratories, Elkhart, IN). The area under the glucose curve was determined as outlined by Wolever13 and previously employed in our laboratory.11 In a preliminary study, both insulin and amylin concentrations were assayed via radioimmunoassay on a similar group of rats in both the fed and fasted states using materials obtained from commercial sources (Penninsula Laboratories, Pomona, CA). Hemoglobin A1c was determined spectrophotometrically via microcolumn resin separation of hemoglobin fractions using commercially prepared reagents and materials (Sigma Chemical Co, St. Louis, Mo).
Data were analyzed via standard statistical procedures with the Statview program (Abacus Concepts, Berkely, CA) with a Powermac 6500 MacIntosh computer and graphics accomplished with the Cricket graph program (Cricket Software, Malvem, PA).
Body weights, adiposity, and glycemic characteristics of LA/Ntul//-cp rats. Body weights of obese rats were significantly greater than weights of their lean littermates, despite consuming the same diets ad libiturn, from weaning. Fasting blood glucose concentrations of obese rats were modestly (approximately 20%) greater than those of lean rats, and the fasting concentrations of insulin and amylin, were significantly greater in obese than in lean littermates. Glycated hemoglobin percentages were similar in both phenotypes, although they tended to be slightly greater in the obese phenotype Glycemic responses to a standard oral glucose load and the corresponding mean glucose area under the curve were significantly greater in obese than in lean animals (Figure 1A) (Figure 1B). Glycemic responses to a glucose load and the glucose area were markedly improved in obese rats after an acute, single-dose oral administration of the FCC complex, with most marked improvement occuring during the post-absorptive phase of the OGT curve, extending from 60 to 120minutes. (Figure 2A) (Figure 2B). Both absorptive and post absorptive areas for the FCC alone were quantitatively similar in both phenotypes, and represented approximately 30% of the glucose area in lean and about 3% of the glucose area of obese rats. In lean rats, when FCC was administered with glucose, the specific 0-120minute FCC-induced reduction in AUCglc averaged 75%, while in similarly-fed obese littermates the total reduction in glycemic responses averaged 33% (Figure 3), consistent with a state of insulin resistance and impaired peripheral glucose uptake in the obese phenotype of this strain.
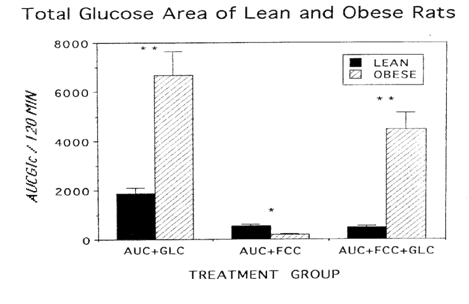
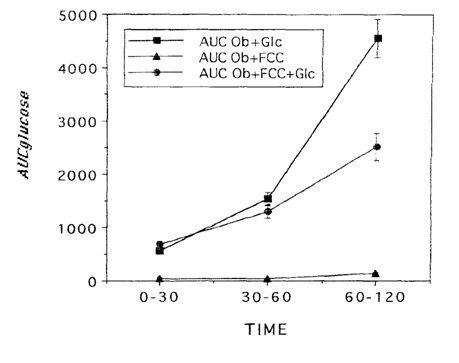
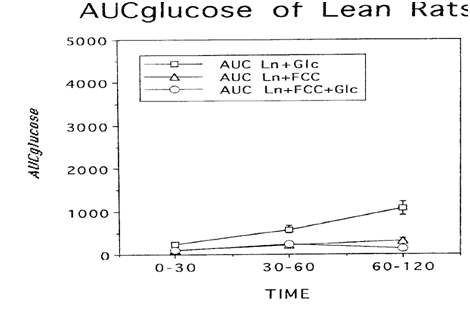
Insulin resistance and impaired metabolic tolerance to carbohydrate ingestion is a common observation in obesity and overweight conditions, and is known to be a risk factor in the pathophysiologic progression of atherosclerosis and lipid disorders of humans. In the congenic LA/Ntul//-cp rat, the impaired glucose tolerance is presumed to be contributory to hper1ipidemia and atherosclerotic lesions which occur later in adulthood, while in the closely related SHR/N//-cp strain, the IGT progresses to NIDDM11 and its attendant sequellae including cataracts,11 impaired thermogenesis15‒17 and severe renal disease.18 Previous studies have reported hypercholesterolemia and hypertriglyceridemia in the obese phenotype of both corpulent strains, combined with exaggerated hyperlipidemic and adipogenic responses to diet,8,19,20 and with impaired thermic responses to diet7,8,12 and environment.7,8 Despite the above complications, obese members of this strain have been observed to survive beyond 30months and lean littermates beyond 48months when fed conventional diets ad libitum.21 Thus, the elements of carbohydrate metabolism of importance to this study are well characterized in the lean and obese phenotypes of the congenic LA/Ntu1//-cp rat strain, and would appear to be similar in nature to characteristics of normal and altered patterns of carbohydrate metabolism which commonly occur in lean and obese humans, respectively.
In the present study, the specific mechanism or mechanisms through which the improvements in glycemic response may have occurred could not be determined, but are presumed to be due to physicochemica1 effects of the frutan polymers in the gastrointestinal tract, combined with more recently discovered effects of chromium on insulin actions.22 Moreover, these actions are likely to occur in varying degrees during both the absorptive and the post absorptive phases of the glycemic response. Fructans and other dietary fibers have been proposed to alter such physiologic functions and parameters as intestinal transit time, water holding capacity and fecal bulk, rates of nutrient absorption, colonic fermentation, increased mucosal proliferation and inhibition of tumor formation5 and others, while chromium is known to exert actions on the insulin receptor, and to enhance the mechanism of glucose disposal in peripheral tissues.22,23 Thus, in the present study, the improvement in the absorptive phase of the OGT is presumed to be secondary to luminal factors including the carbohydrate-water holding characteristics of the fibers and resulting in an attenuation of gastric emptying. In contrast, the substantially greater improvement in glycemic response which occurred in both phenotypes during the post absorptive phases of the OGTs are presumed to be due to the presence of chromium, an essential component of glucose tolerance factor (GTF).22,23
The effectiveness of insulin has been shown to be greater in the presence of chromium, where it has been proposed to enhance insulin binding and action, and in an activation of the insulin receptor.22,23 In a clinical trial, glycemic control in 118 of 162 insulin-dependent diabetic subjects became improved following supplementation with chromium.24,25 The molecular forms of the chromium in the present study were as chromium dinicotinate and chromium picolinate (50-50 mix), both of which have favorable absorption in the small intestine, and both of which have demonstrated effectiveness in overall glycemic control.22,24 The more rapid glucose disposal in lean than obese animals is consistent with significantly greater insulin resistance and impaired peripheral glucose uptake reported in obese than in lean rats of this strain.9,11 The effects of chromium may have partially overcome the insulin resistance of the obese animals, and resulted in an attenuation in the glycemic responses observed.
The inclusion of fructose in the dietary wafer formulation studied significantly improved palatability, but is presumably without significant physiologic consequence because of the limited quantity provided in the wafers. Fructose has been shown to enter tissues via diffusion or facilitated diffusion via the GLUT-5 transporter, and thus does not require the presence of insulin to effect membrane transport and uptake in tissues.2,26 Once internalized, fructose can undergo glycolytic activity readily, but when consumed in moderation it is without apparent detrimental effect on the mammalian organism. As a natural constituent of many fruits and vegetables, fructose has always been a modest constituent of the human diet. In abundance, however, fructose ingestion (equal to 27% of diet w/w) resulted in an exaggeration of the hyperlipidemia and hyperinsulinemia of obese diabetic SHR/Ntul//-cp rats, and in a further deterioration of glycemic control.27,28 The comparison between the LA/Ntul//-cp and SHR/Ntul//-cpstrains lends additional strength to the observation, in that both strains carry the same genetic trait forobesity, transmitted in an identical fashion, but in different genetic background strains (i.e., the LA/N vs the SHR/N strains). In summary, the administration of FCC resulted in a marked and significant improvement in glycemic response to a standard glycemic challenge, and which may contribute to enhanced glycemic control and a decreased magnitude of glycemia-related pathophysiologic sequellae if continued over time.29
In summary, the results of this study further confirm the obese and glucose intolerant status of the LA/Ntul//-cp rat strain. The effect of the dietary supplement DahluleanTM which consists predominantly of a fructan polymers in combination with chromium dinicotinate glycinate and chromium picolinate, resulted in a partial normalization of the glycemic response in genetically homogenous (congenic) obese, glucose-intolerant rats as measured by a standardized Oral Glucose Tolerance test and followed by computation of the sequential glucose area which resulted there from. Reduction of the glycemic responses of meals toward normal is a highly desirable attribute of treatment regimens for obesity and overweight conditions, and is likely to result in a reduction of the metabolic and pathophysiologic sequelae which often progress with those conditions in both man and animals. While the specific biochemical mechanisms through which the improvements in glycemic response may have occurred cannot be determined from the present study, it is presumed that they were due to the combined effects of the fructans and the chromium on luminal factors of gastrointestinal transit, nutrient absorption, and cellular uptake of glucose in peripheral tissues. In conclusion, fructan-chromium complex as formulated in DahluleanTM would appear to be a useful nutraceutical in the control of post-prandial glycemia, and as such, a useful adjunct in the dietary treatment of obesity and glucose intolerance secondary to insulin resistance, prediabetic, and diabetic states.30
The authors wish to thank Ms. Tracy Scipio, University of Science, Arts, & Technology, Montserrat for assistance in the preparation of this manuscript; Dr. S. Dubin and Ms. M. Victor, Drexel University Animal Resources, for animal husbandry support; Ms. Susan Krasner, B.S., for assistance in data collection, and HPF-LLC, of Trevose, PA for their generous gift of DahluleanTM. Supported by Institutional resources and project grant DU426001093, and Institutional Resources of the University of Science Arts and Technology, Montserrat, B.W.I., Postal code MSR1110.
Author declares that there is no conflict of interest.

©2016 Tulp, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.