Journal of
eISSN: 2469 - 2786


Research Article Volume 11 Issue 2
1Laboratories, Indoor Environmental Consulting and Labs (IECL), Australia
2Consultancy, Indoor Environmental Consulting and Labs, Australia
Correspondence: Dr. Alexander D Wilkie, IECL, 5/158 Murarrie Road, Murarrie, Queensland, Australia
Received: July 24, 2023 | Published: August 7, 2023
Citation: Wilkie AD, Letters S, Venz L. Issues with percentage coverage-based non-viable fungal analysis and new methodology for enumeration-based analysis. J Bacteriol Mycol Open Access. 2023;11(2):104-108. DOI: 10.15406/jbmoa.2023.11.00353
The choices of methodology for analysis of non-viable fungal surface samples are yet to be agreed upon. The two main types of analysis of non-viable fungal (gross fungi) samples can be generally categorised as counting based (enumeration of fungal structures) or categorization based (such as percent coverage estimates or fungal rating categories). We present evidence of flaws in the percent coverage methodology due to a strong dependence on individual analyst’s subjective estimates. By using image analysis and a survey of analysts we show the high variability between analysts’ percent coverage estimates. We also propose an enumeration-based methodology which attempts to address problems in current counting-based methods by using a semi-random grid pattern of fields of view rather than a traverses-based approach.
Keywords: fungal analysis, non-viable, surface sampling, bio-tape, tape-lift, gross fungi, gross fungal, percent-coverage, enumeration, semi-random fields, image analysis
ASTM, American society for testing and materials; FS, fungal structures
Mould growth in the indoor environment continues to be a problem for health and wellbeing of occupants.1,2 Non-viable fungal sampling can provide useful information in the identification of hazards in buildings,3,4 as visual inspections of premises for fungal contamination can potentially miss sources of mould contamination.5 Effective monitoring of mould remains vital as mould exposure has been linked to adverse health reactions.6–12
The majority of sampling for fungal contamination of buildings in Australia is conducted using non-viable sampling techniques. Non-viable sampling remains the preference over other methods of fungal sampling due to the low cost and time effectiveness.13 Additionally, non-viable sampling can detect the presence of both viable and non-viable mould- both of which have been indicated as potential allergens.1,10,14–16 Fungal fragments have been identified as potential contaminants,17,18 and inactive fungal material still retains the capacity to release toxins.19
Non-viable fungal sampling is somewhat of a misnomer, in that it is not detecting solely non-viable fungi (i.e., dead fungi). It is rather detecting both live and dead fungi – fungi that grow in culture, fungi that do not grow in culture but are still viable (Viable But Non-Culturable (VBNC)),20 and fungi that is not viable. Terms like ‘total fungal spores’ or ‘total spore count’ have also been used to describe such fungi21 -but the term 'spore’ does not cover all fungal material and implies viability in many accepted definitions of the word. In this article we shall henceforth refer to non-viable fungi (including culturable, VBNC and non-viable fungi) as “gross fungi”.
Visual inspection
Visual inspection of buildings for fungal contamination remains a key part of determining the extent of contamination in structures. Trained inspectors should be able to identify fungal growth with decent reliability through visual inspection. In a recent paper, image analysis paired with visual mould inspections were used to quantify mould growth in structures.22 Visual inspections for determining mould contamination of buildings are unlikely to cease for the foreseeable future.
Why use microscopy when molecular techniques exist?
Microscopic analysis for fungi provides a method of determining the extent of mould contamination in a cost-effective manner. While it cannot provide information on the species of fungi present, it can provide information on the presence of water damage, if air quality is being affected by a hidden mould issue or if efforts to remediate a premises have affected fungal levels.3,5,23 Data from gross fungal sampling of premises should be paired with outdoor reference data from the local area to aid in identification of mould issues.24 Use of microscopic analysis of gross fungi samples for fungal structures such as conidiophores, can also provide insight about the activity of mould in samples, whereas molecular based techniques do not.25 Time limitations and costs of molecular techniques have delayed the adoption of these methods -however they provide useful data to aid in the medical care of patients suffering from mould exposure.26
Surface sampling for gross fungi is typically conducted using tape-lift samples23,27,28 (e.g. Zefon Bio-Tape) in a technique which was first described by Flegel (1980).29 Tape lift samples are popular for gross fungal samples due to the ease of use, repeatability of sampling, ease of analysis and cost effectiveness. Non-viable mould samples are analysed by microscopy with the use of either phase contrast or stains to improve contrast.30 While multiple methods for analysis of gross fungi surface samples have been described23 reliable quantitative methods are generally considered to be of greatest use.31 A quantitative method of analysis of tape lift samples has recently been published showing that quantification of fungi using enumeration is achievable.31
Improvements in efficiency, consistency and reliability of data obtained from microscopic analysis of fungi remain important to cost and time effective analysis of gross fungal sampling. This article aims to improve accuracy, comparability and efficacy of gross fungi surface sample analysis to help laboratories improve consistency and efficiency by highlighting issues in percent coverage-based techniques and updating enumeration-based techniques. Here, we investigate techniques commonly used in the analysis of tape-lift samples through image analysis and a survey of analysts and propose an enumeration-based method of analysis that provides benefits with regard to bias as compared to currently accepted enumeration methods.
Proposed methodology for analysis of fungal tape-lift samples (Semi-random field counting method)
Notes
Image analysis
Reference images for the ASTM D7658 standard for estimating percent coverage were analysed using NIS elements basic research software. Analysis for percentage coverage of images with debris was achieved using the software’s ‘object count’ function and defining gates of material to be counted (e.g. RGB thresholds, object diameter). The software can then determine percentage coverage of the selected objects in the image. Figure 1 shows an example of the software counting an image.

Figure 1 Percentage coverage of image 3 from Figure 1 in ASTM D7391 [33] by image analysis software. Analysis was performed using NIS elements software ‘objects count’ function.
a) original image, b) image analysis example. Selected objects are outlined in green. Measured area as a percent of the total image is indicated in red.
Survey for estimates of percent coverage
A small survey was conducted of laboratory personnel estimates of the percent coverage of the ASTM reference images (N=7). These results were then compared to the stipulated ASTM ranges for each image, and the image quantification measurement derived from the software analysis.
Statistical analysis
Mann-Whitney U tests were performed using Statistics Kingdom online software (Statistics Kingdom)32 and Boxplots were generated using Microsoft Excel (Microsoft Inc.). Mean, standard deviation and interquartile ranges were calculated. Separate experiments were performed N number of times as listed in each figure and table.
Use of image analysis software on the example images provided in the ASTM D7391 standard33 revealed that the actual measured debris loading did not match with the ranges indicated. It should be noted that the same images are used in both ASTM standards D7391 and D7658.30,33 The measured values for images are shown in Table 1. Of the six images (Figure 3) three of the measured values were not in the designated ranges at all (images 3,4,5) (Figure 2); two were at the bottom 1% of the range indicated (1,2) (Figure 2); and one matched (0) (Figure 2) – however this image did not have any debris present to quantify and may not be relevant data. It should also be noted that the images from ASTM D7391 contained numbers in the top left of each image which were counted by the software and may cause slight positive bias to the measurement. However, from the quantification of the numbers in example images ranging from 0.37-0.61%, the positive bias to the calculation is unlikely to exceed 1% of each image.
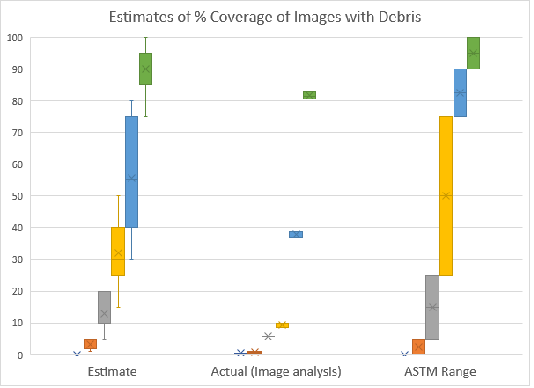
Figure 2 Comparison of percent coverage estimates from ranges listed in the ASTM documents, image analysis, and the survey of analysts’ estimates. For image analysis 3 separate readings were collected (N=3). For the Estimate, seven people were surveyed (N=7).
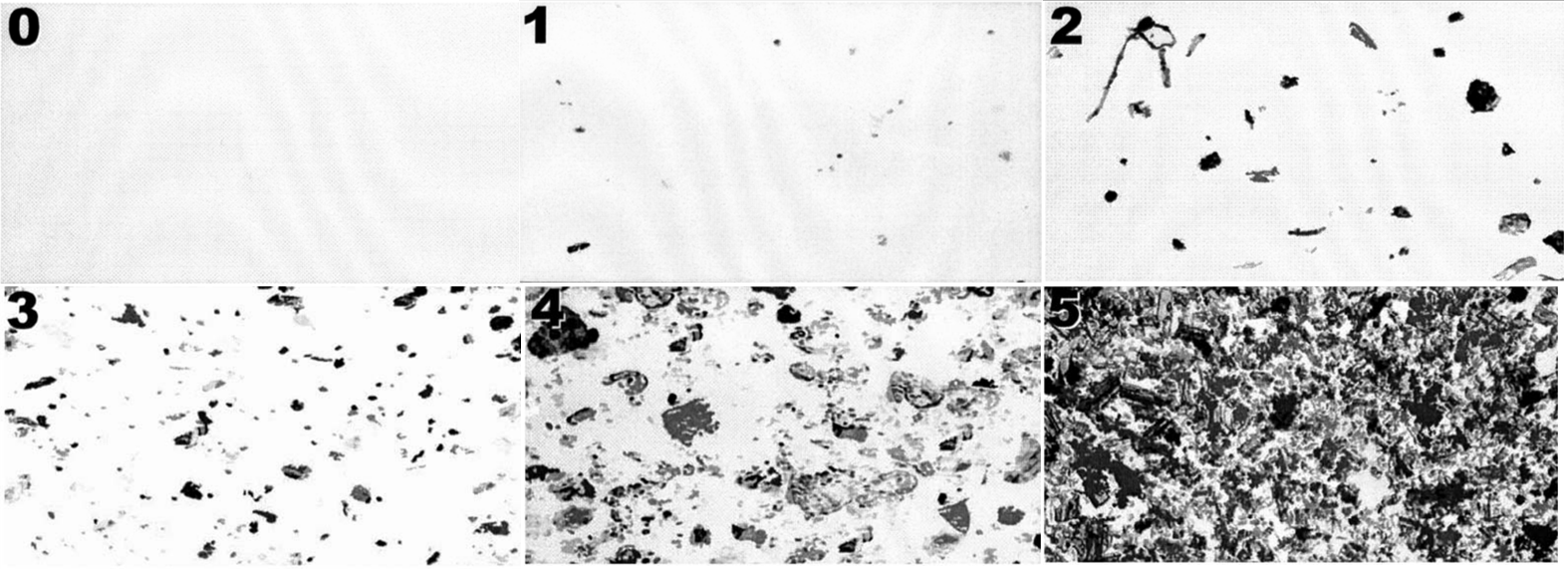
Figure 3 Example images from Figure 1 in ASTM D7391 and D7658 representing debris loading of samples.
Image |
ASTM indicated range |
Image quantification measurement (average) |
Survey estimates (average) |
0 |
0% |
0.55% |
0% |
1 |
>0–5% |
0.82% |
2.86% |
2 |
5–25% |
5.79% |
11.50% |
3 |
25–75% |
9.36% |
28.50% |
4 |
75–90% |
38.02% |
49.25% |
5 |
90–100% |
81.64% |
79.38% |
Table 1 ASTM ranges for debris coverage compared to image quantification measurement
Additionally, a small survey of analysts asked to quantify percent coverage of the images did not provide consistent results as compared to either the values measured by image analysis or the ranges listed in the ASTM document (Figure 2). Notably, a Mann-Whitney U test comparing the mean values of the survey results and image analysis, showed significant differences in the mean percent coverage for image 3 (p=0.02225) (Table 2). Furthermore, very high variability was observed in the estimates of percent coverage of the images by analysts. Finally, it should be highlighted that this image analysis function; requesting analysts to quantify percent coverage, is based on a single field of view and does not account for error in analysts both quantifying percent coverage, combined with how much of that is fungal material in the sample. Figure 2 shows data from the percent coverage ranges listed by the ASTM compared to image analysis measurements, and the small survey of estimates (N=7).
Image |
Image analysis (% Coverage) |
Survey estimates (% Coverage) |
P value |
||||||||
0 |
0.53% |
0.56% |
0.55% |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0.005 |
1 |
0.63 |
0.87% |
0.96% |
5 |
2 |
1 |
5 |
5 |
2 |
2 |
0.0195 |
2 |
5.74 |
5.84% |
5.78% |
20 |
10 |
5 |
20 |
15 |
10 |
10 |
0.1052 |
3 |
8.65 |
9.94% |
9.5% |
30 |
15 |
30 |
40 |
35 |
50 |
25 |
0.0223 |
4 |
38.6 |
38.78% |
36.68% |
50 |
30 |
60 |
80 |
55 |
75 |
40 |
0.1167 |
5 |
81.4 |
82.9% |
80.61% |
90 |
85 |
95 |
95 |
90 |
100 |
75 |
0.1084 |
Table 2 Data from survey of estimates for percent coverage of images and image analysis. Image quantification of the representative images from ASTM D7391 Figure 1 was conducted using NIS elements ‘object count’ function, 3 times per image (N=3). A small survey was conducted asking participants to quantify the percent coverage of the images from ASTM D7391 Figure 1 (N=7). P values from Mann-Whitney U tests for each image comparing image analysis to survey estimates are shown
Many of the proposed methodologies for fungal analysis use techniques based on percent coverage of slides.23 Methodologies based on percent coverage are prone to bias due to the exceedingly subjective nature of such estimates. For example, in the ASTM standards,30,33 the example images demonstrating percent coverage did not coincide with our data from image analysis or the surveyed estimates (Figure 2). Image quantification of the ASTM images showed values either outside the suggested range of percent coverage or at the limits of the ranges indicated (Table 1).
Our survey of analysts’ estimation highlights the variability between analysts, and inaccuracy when compared to image analysis (Figure 2). Additionally, in the ASTM standard, analysts are asked to quantify not only coverage by debris, but to separate fungal structures from other debris for quantification -quite a complicated image analysis function to be performed by a human. Furthermore, for sample analysis, analysts must either select an image which they feel is representative of the sample, or combine multiples fields of view in their mind to estimate fungal loading –both of which may introduce bias. All the factors listed here lead us to the conclusion that an estimate of percent coverage is heavily predicated on individual analysts, and likely not an accurate representation of the data presented from a tape lift slide.
The percent coverage technique was not designed for quantification of fungal material. It was originally used in environmental science to estimate vegetation coverage,34 adapted or independently developed in geology for estimating mineral content in sedimentary rocks,35 modified for asbestos coverage estimation,36 used for estimation of debris in microscopy images,30,33 and finally to percent coverage of fungi in photomicrographs.30 In these prior applications it should be highlighted that quantification of structures was often not the primary purpose of the techniques, raising the question whether percent coverage is an appropriate method for the quantification of fungal structures in microscopy images. For example, in Plant Sociology34 in the percent coverage estimation of canopy cover, the Braun-Blanquet method is not attempting to give a rating on the number of leaves and branches present, but assessing how much of the sky the tree canopy blocks. Using the fungal loading categories listed in ASTM D7658 as an example –these categories would make no distinction in the mould content of a sample entirely covered with large Alternaria spores as compared to a sample covered with small Aspergillus spores; whereas a counting based approach may indicate orders of magnitude in difference between the spore counts of different mould genera in samples.
Others have indicated that counted and calculated values should not be used due to inaccuracy of counting the results.37 However, attempts to quantify mould on surfaces without the use of counting and numbers is at least just as inaccurate, and arguably far more so. In order to supply useful comparable results, a scale must be used –either based on counting of material present, or categorisation based on any individual analyst’s interpretation of the percentage of mould coverage in a sample. Counting based techniques start from a similar footing – counting of individual spores, and any variation in analysis stems from incorrect identification of the indicated base unit. Alternatively, categorization-based methods rely on subjective interpretations of the material which may vary considerably depending on an individual’s training, background and thought processes. In this approach the categories used may be very broad or ambiguous, which may limit their usefulness in attempting to compare samples.
In contrast to counting based techniques, authors have stated “Laboratory reports should not state results in terms of number of spores per unit area, because the measure is meaningless”.37 However, as they do not elaborate on this comment, it is open to interpretation. It may have been meant to say that the FS/cm² taken from one area of a surface may not be representative of the surface as a whole – which is entirely reasonable; not that any numerical indication of surface fungal content is useless. The authors of this document make many other useful comments, especially in recommending surface mould sampling should examine for the presence of growth and sporulation structures.37
In order to provide the most reasonable and accurate representation of mould content on a surface the sources of bias must be considered. Sources of bias common to both counting and percentage-coverage based methods are focused on the methodology of collecting the samples –such as pressure used in collecting the sample, roughness of the material being sampled or scraping the sample across the sample rather than pressing.38 Analyst bias is also a factor in counting based methodology, however misidentification of individual spores or analysts miss-counting spores is likely to produce small variations as compared to analysts estimating coverage –which is by definition not quantifiable and may potentially cause large variations. Sources of bias in counting fungal surface samples by microscopy have generally been accepted to include: analyst fatigue, analyst misidentification of spores, spores hidden by debris or other material, and spores missed by analysts (e.g., small, hyaline).4,30 Sources of bias in estimating percent coverage or other ratings-based techniques include: analysts thought processes on how to quantify images, analyst fatigue, analysts’ memory and aptitude in combining multiple microscope fields of view in their mind, analysts estimating percent coverage of both fungal material and debris simultaneously, and analyst bias from viewing few or one heavily contaminated or clean fields of view.
To limit these biases and potential sources of error, we propose the semi-random field counting method; the quantification of mould structures in semi-random fields spread across the sample in a grid pattern of the tape-lift. This method may be more effective compared to other analysis techniques for several reasons. Firstly, using traverses may increase bias if a sample has heavy mould growth along one edge. Secondly, spreading the fields viewed across the surface should minimise conscious or subconscious bias of analysts to either find or avoid areas of high mould growth. Due to these factors, it provides the best opportunity for the most accurate representation of the material present on the slide without requiring the entire sample be analysed.
Further, while an initial visual scan of the slide at low magnification may be useful in determining debris loading and areas of interest – it is our view that it is more beneficial to either skip this step or conduct it after analysis at 400x or higher has been performed. Having the analyst examine the entire slide before quantification may cause bias – especially in the selection of semi-random fields. Additionally, while low magnification can sometimes be used to identify fungi – it is far less reliable and consistent – especially on small or hyaline spores.4
It should be noted that inaccuracies caused by reading less than the entire tape lift sample are extremely difficult to avoid completely. Due to the clustering nature of mould growth and uneven distribution on slides calculated counts of fungi on a surface are unlikely to perfectly coincide with the actual count from the entire slide. However, they provide a reasonable representation of the mould content on each slide, in a manner that makes comparison with other samples simple. While small differences between samples would not provide useful comparative data, larger differences provide a meaningful basis for further investigation.
In this article we have addressed potential issues with percent coverage-based analysis techniques. Our data has demonstrated that coverage estimates vary considerably, are difficult to compare – even to representative images, and that even widely accepted reference images may not be good representations of data when tested by image analysis software. This data highlighting potential issues in percent-coverage based techniques may help address the quality and comparability of gross fungi analysis data. We have also presented an argument for a counting based methodology of gross fungal tape-lift sampling designed to reduce bias.
Use of semi-random fields in a grid pattern eliminates the need for set sampling areas required for traverse based counting. Use of the semi-random field counting method would shift the focus away from analyst estimate bias, towards a focus on the data. Finally, as mentioned previously it avoids bias from deposition of fungal material in gradients or clusters. While counting-based analysis techniques of gross fungi samples have limitations, they will likely be more consistent and comparable as compared to percent coverage-based estimation methods. This methodology addresses some issues with enumeration of gross fungi which helps to provide access comparable information for use in building inspection.
Finally, we have proposed the term “gross fungi” which articulates a clearer meaning than “non-viable” or “total fungal spores” for future use.
Analysis of our proposed methodology in detail as compared to samples read in their entirety may provide insight as to how many random fields provide the most accurate representation of the surface using the least amount of analyst’s time. Additionally, percent coverage techniques should investigate the use of image analysis software to minimise analyst bias in estimates.
The authors would like to thank IECL and NLR Restorations for funding this research.
The authors declare that there are no conflicts of interest.

©2023 Wilkie, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.