MOJ
eISSN: 2381-182X


Mini Review Volume 7 Issue 2
Ecologie végétale, Département de Biologie, Tunisie
Correspondence: Olfa Rbia, Ecologie végétale, Département de Biologie, Faculté des Sciences de Tunis, Campus universitaire 2092, El Manar II, Tunisie
Received: October 04, 2018 | Published: March 21, 2019
Citation: Rbia O, Smiti SA. Therapeutic potentialities of prickly pear Opuntia spp. MOJ Food Process Technol. 2019;7(2):39-42. DOI: 10.15406/mojfpt.2019.07.00217
Opuntia spp. is an important cactaceae inhibiting and preventing some pathologies. Thus, in the present mini review, a short focus is drawn to the therapeutic potentialities of Opuntia bioactive compounds. In fact, this review aimed to provide sufficient background informations about some biological activities of Opuntia spp. such as antioxidant, neuroprotective, anti-inflammatory and antidiabetic activities. In order to give a solution for some pathologies, a special emphasis is paid to biological mechanisms of Opuntia fruits, stems and oils biomolecules.
Keywords: neuroprotective, anti-inflammatory, Opuntia, hypoglycemic, inflammations
The culture of Opuntia spp. is practiced, in certain countries such as Mexico, Spain or Italy, in an intensive and modern way with programs of research and development for the production of fruits or fodder and even for nutraceutical, cosmetic and pharmaceutical industrial uses.1‒3 The medicinal use of the Opuntia translated by the use of various parts of the plant. The fruits are known everywhere in Morocco by the fact that they stop the colics and the diarrheas.4 In Australia and South Africa, the hypoglycemic effect of Nopalitos is used for the treatment diabetes nondependant on insulin.5 The mucilage isolated from the cladodes allows to reduce total cholesterol in blood. The cladodes are used to treat inflammations.6
Anti-oxidant activity of Opuntia ficus indica
Antioxidant activity of the prickly pear fruit juice extract: A Moroccan study evaluates the antioxidant activity of prickly pear fruit juices, in vitro, by the DPPH•test. The chemical compound 2,2-diphenyl-1-picrylhydrazyl was one of the first free radicals used to study the structure-antioxidant relationship of phenolic compounds.1‒3 It has an unpaired electron on an atom of the nitrogen bridge (Figure 1).
DPPH • purple in color, turns yellow in the presence of free radical scavengers, and is reduced to 2,2-diphenyl-1-picryl hydrazine.
The results obtained showed that phenolic compounds, flavonoids and betalain-type pigments have greater antiradical activities than vitamin C. The raw juices have higher activities than the compounds that constitute them. The purple-colored juices have higher anti-oxidant activity than orange-yellow fruits.1
Antioxidant activity of the prickly pear: Another study, performed on isolated and purified polysaccharides, extracted from Opuntia ficus indica grown in China, demonstrated the antioxidant activity of the latter. A reduction, dependent concentration, of the formation of the superoxide radical and the hydroxyl radical has been asserted. Polysaccharides (rhamnose, arabinose and glucose) can effectively prevent the hydroxyl radical produced by the fenton system. The Fenton reaction is based on the production of radicals from the decomposition of hydrogen peroxide catalyzed by ferrous salts.4
The study also showed that Opuntia ficus indica polysaccharides prevent the regeneration of malondialdehyd (MDA) in the microsome of mice liver. Malondialdehyd is one of the end products of the peroxidation of polyunsaturated fatty acids in cells. An increase in free radicals causes overproduction of MDA. The level of malondialdehyde is commonly known as a marker of oxidative stress and antioxidant status in cancer patients. Oxidative stress results from an imbalance between the production of oxidant elements and antioxidant defense mechanisms that comes either from an exaggerated production of oxidant agents or from an alteration of the defense mechanisms. When one or other of these mechanisms is present, oxidative stress is initiated and contributes by its multiple consequences on nucleic acids, proteins or lipids, to the pathogenesis of certain diseases such as cardiovascular diseases, neurodegenerative diseases or cancer.7,8
Antioxidant activity of the prickly pear oil: Antioxidant seed oil activity was assessed by means of DPPH radical-scan analysis and the carotene bleaching test. Both methods demonstrated significant anti-oxidant activity of prickly pear seed oil, comparable to ascorbic acid and butylated hydroxytoluene. Antioxidant activity of seed oil was also found concentration dependent.9,10
Neuroprotective activity of Opuntia ficus indica
Recently, the methanol extract of Opuntia ficus indica fruits varieties has shown significant efficacy in the fight against neuronal damage induced by free radicals in cortical cultures of mice.
Given the roles of the oxidative and free radical effects in neuronal death after ischemia and in neurodegenerative disorders including Alzheimer's disease, Korean researchers have attempted to identify the active ingredients of the prickly pear and to characterize their neuroprotective and anti-oxidant actions using rat cortical cells.
Among the isolated constituents in the fruits and stems of Opuntia ficus indica varieties, the three flavonoids: quercetin, dihydroquercetin, and quercetin 3-methyl ethers have been effective in protecting rat cortical cells.
The cultures were prepared from the cerebral cortex of rat embryos. Neuronal damage was caused by caspase3. Aspartic acid-specific cystein proteases are a group of cysteine proteases that play a key role in apoptosis, necrosis and inflammation. Of the three flavonoids, quercetin 3-methyl ether is the most effective in the fight against neurotoxicity.
It is clear that the neuroprotective actions of Opuntia ficus indica appear to be active for the prevention and treatment of neurological disorders caused by oxidizing agents.11,12 The methanol extract of Opuntia ficus indica also has a neuroprotective action against N-methyl-D-aspartate NMDA, kainate KA and OGD oxygen deprivation oxygen, inducing neuronal alterations in cultures of mouse cortical cells. The evaluation of this protective effect of methanol extract of Opuntia has also been studied in the hippocampal region CA1, against the neuronal damage evoked by global ischemia in gerbils. Treatment of neuronal cultures with methanol extract of Opuntia ficus indica (30, 300 and 1000 μg/ml) inhibited NMDA and OGD inducing dose-dependent neurotoxicity. The extract appears to significantly reduce neurotoxicity-induced NMDA with a 27% level in gerbils treated with Opuntia methanol every 24 hours for 3 times daily for 4 weeks. Preventive administration of Opuntia ficus indica is probably useful in the relief of neuronal damage caused by global ischemia.13
Anti-inflammatory activity of Opuntia ficus indica
An anti-inflammatory action has been shown in the ethanol extract of the Opuntia ficus indica cactus grown in Korea. After fractionation of the methanol extract of the cactus stems, an active anti-inflammatory principle was isolated and identified, β-sitosterol. Sistosterol, although its activity seems to be relatively lower compared to that of hydrocortisone, seems to be the main cause of the anti-inflammatory activity of Opuntia ficus indica.6
Antidiabetic activity of Opuntia spp
Antidiabetic activity of Opuntia fuliginosa: A study14 on the evaluation of the hypoglycemic activity of a purified extract of Opuntia fuliginosa cladodes in Streptozocin-diabetic rats showed that glycemia and glycosylated hemoglobin were reduced to combined treatment with insulin and Opuntia fuliginosa extract. When insulin was removed from the combined treatment, the single prickly figure 2 extract maintained euglycemia in diabetic rats. Glucose response to glucose administered also showed that rats receiving the combination therapy of insulin and Opuntia cladod extract for 7 weeks followed by the extract alone were able to rapidly adjust blood glucose levels. At the level of that of non-diabetic rats.
The same study reports that in humans, consumption of cladode extracts resulted in weight loss. Diabetic rats receiving Opuntia fuliginosa extract maintained a steady body weight, while weight loss was observed in untreated diabetic rats. The hypothesis proposed by Frati Munari et al.,15 is that cladode extract enhances glucose utilization at the cellular level.
The control of diabetes by the purified extract of cladodes of Opuntia fuliginosa can not be explained by its action as a dietary fiber, since several long-term studies have led to the conclusion that the different soluble fiber sources do not lower or adjust blood glucose.16,17
Knowing also that the level of glycosylated hemoglobin in diabetic rats that received Opuntia fuliginosa extract or a combination of insulin and Opuntia fuliginosa extract returned to normal values after the eighth week, while diabetic rats treated with insulin, only the level of glycosylated hemoglobin has not been effectively brought up to standard. The extract of Opuntia fuliginosa is presumed to function as a coadjutor of insulin.18
In addition, pancreatectomized rabbits fed on prickly pear stems showed low blood glucose levels.18 The subcutaneous glucose tolerance test also shows that insulin and Opuntia fuliginosa extract administered to rats show a significant drop in blood glucose with normal blood glucose levels within 120 minutes. Among others, non-insulin-dependent diabetic patients receiving fresh or cooked stems of prickly pear showed lower levels of glucose but no hypoglycemic effect.19 Another important finding of the present study is that control of diabetes with a purified extract of Opuntia fuliginosa can be achieved with daily oral doses in the range of 1 mg/kg body weight.20
Antidiabetic activity of Opuntia monacantha: Other researchers have investigated the effect of polysaccharides extracted from cladodes of Opuntia monacantha (POMC) on carbohydrate metabolism in streptozotocin diabetic rats. They compare its action with dimethylbiguanide, by determining blood glucose, total cholesterol, total triglyceride and the level of HDL cholesterol; polysaccharides extracted from cladodes have shown beneficial effects on the improvement of lipid and blood sugar levels. Daily treatment at 100-300 mg/kg of POMC for four weeks not only brought a significant decrease in blood glucose level of diabetic rats, but also increased the level of HDL cholesterol.
Compared with groups of rats treated with dimethylbiguanide, the mechanism of action of POMC could be similar. The level of insulin in diabetic rats was not significantly affected by the treatment of POMC and dimethylbiguanide from which it can be concluded that polysaccharides reduce intestinal glucose uptake, enhance insulin sensitivity by increased uptake and peripheral use of glucose and decreased hepatic glucose production by inhibiting gluconeogenesis and glycogenolysis.21‒23
Antidiabetic activity of Opuntia dillenii: Another study investigated the antidiabetic effect of polysaccharides of Opuntia dillenii in mice rendered diabetic by strepotozotocin. The oral administration of polysaccharides decreased significantly: taking food and water; glycemia; the total cholesterol level; the triglyceride level; the MDA rate and the activity of glucose-6-phosphatase (G-6-Pase). On the other hand, there has been a remarkable increase: body weight; hepatic glycogen level; the level of high density lipoproteins; hepatic superoxide dismutase; and glutathione peroxidase.
However, insulin levels did not increase significantly in mice with induced diabetes. The study proposes the hypothesis that Opuntia dillenii polysaccharides exert its anti-hyperglycaemic effect by protecting the liver against peroxidation damage and maintaining tissue function, thereby improving the sensitivity and response of target in insulin-diabetic mice.24
Antidiabetic activity of Opuntia humifusa : A more recent Korean study25 investigated the effect of administration of Opuntia humifusa stem extract (OHSt) on diabetic rats (type I diabetes). The observation of the effects did not concern only glycemia and lipidemia but also on the cells and the liver enzymes and the pancreatic tissues.
Forty six-week-old rats divided into 5 groups:
The stems of Opuntia humifusa powder have been suspended in distilled water and are administered by a gastric tube. After 7 weeks of treatment, the blood glucose and triglyceride levels of cladode extract-treated groups were significantly lower compared to the group of diabetic rats. The treatment also resulted in a significant decrease in total cholesterol and low density lipoprotein, accompanied by a significant increase in high density lipoprotein. In addition, the levels of alanine aminotransferase and aspartate aminotransferase were significantly lower in rats in the stem-treated group than in the untreated diabetic group. A significant increase in pancreatic cell volume of rats treated with 500 mg/kg of oral extracts was reported. The histological results of the pancreatic tissues by immunohistochemical test of the treated rats are positive for the insulin antigen for most of the Langerhans beta cells (Figure 2).25
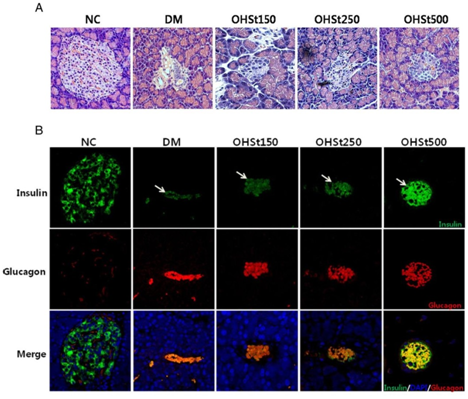
Figure 2 (A) Histological appearance of Langerhans islets of the pancreas of the different groups of rats (stained with hematoxylin and eosin), (B) Confocal microscopic representation of insulin (green stain) and glucagon (red stain) and both (orange to yellow stain) in immunohistochemical tests carried out on the pancreas of treated rats.12
Overall results suggest that stems of Opuntia humifusa have potential hypoglycemic and hypolipidemic activity in diabetic rats.25
These data highligts the great importance of valorizing Opuntia spp as a source of natural bioactive compounds with high therapeutic potentialities that can be used in cosmetic, nutraceutical and pharmaceutical industries.
None.
Author declares that there is none of the conflicts.

©2019 Rbia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.