MOJ
eISSN: 2381-182X


Research Article Volume 7 Issue 3
Department of Nutrition and Food Science, University of Valencia, Spain
Correspondence: Esteve MJ, Department of Nutrition and Food Science, University of Valencia, Av, Vicent Andrés Estellés, s/n. 46100 Burjassot. Spain, Tel +34 963544955, Fax +34 963544916
Received: August 27, 2019 | Published: September 9, 2019
Citation: Carbonell-Capella JM, Blesa J, Frígola A, et al. Study of the interactions of bioactive compounds and antioxidant capacity of an exotic fruits beverage that sweetened with stevia. MOJ Food Process Technol . 2019;7(3):79?86. DOI: 10.15406/mojfpt.2019.07.00224
A functional beverages, with a high content in bioactive compounds, based on exotic fruits (mango juice, papaya juice and acai) mixed with orange juice, oat and sweetened with different concentrations (0, 1.25 and 2.5%w/v) of Stevia rebaudiana (Stevia) extracts, a natural source of non-caloric sweeteners (steviol glycosides) were formulated and studied. Ascorbic acid, total carotenoids, total phenolics, anthocyanins, total antioxidant capacity (TEAC and ORAC methods) and steviol glycosides were evaluated. Beverages sweetened with 1.25 and 2.5% (w/v) Stevia showed a significant increase (3-fold and 4-fold higher, respectively) in phenolic compounds as well as in antioxidant properties in comparison with the beverage without Stevia. The displayed results indicate the potential use of Stevia as an alternative non-caloric sweetener in the preparation of beverages based on fruit juice mixtures and oat, as it may provide good nutritional and physicochemical properties and also enhance the already existing beneficial effects of fruit juices. Synergistic interactions observed between phytochemicals and steviol glycosides in the complex food beverages when TEAC method was used suggest an improved solubility or stability of antioxidant compounds and hence, the combined antioxidant capacity measured by TEAC assay is potentiated in the complex food matrix.
Keywords: exotic fruits, stevia rebaudiana, bioactive compounds, antioxidant capacity, interaction factor
During the last decade, research dealing with health promoting features of so-called functional foods is increasing, with a promising future and a challenging development. A change in food consumption trends is observed, mainly due to lifestyle changes. Proportionally, juices obtained from exotic fruits are the group with the highest growth rate, both in Europe and in North America.1 In terms of public health, drinking fruit and vegetable juices may well be as effective as consuming whole fruits and vegetables with regard to reducing the risk of chronic disorders.2 Many fruits including acerola, acai, avocado, durian, kiwi, mango, papaya, oranges, etc., have attracted much attention because of their health benefits due to the wide range of bioactivities,3 containing a large quantity of bioactive compounds such as ascorbic acid, phenolic compounds and carotenoids, which have been shown to be good contributors to total antioxidant capacity of foods.4,5
Meanwhile, acai (Euterpe oleracea) berry, native of Brazil, has been acclaimed to have a wide range of health-promoting and therapeutic benefits due to its reportedly high levels of antioxidants, with a relatively high content of polyphenols, mainly anthocyanins.6 Findings demonstrate that acai pulp improves biomarkers of physiological oxidative stress.7
Furthermore, the use of natural green plant extracts or their derived products in foods and beverages is also becoming an increasing trend in food industry.8 Stevia rebaudiana, an herb native to South America, is used as a natural source of non-caloric sweeteners (steviol glycosides) and is thought to possess antioxidant, antimicrobial and antifungal activity.9 Although Stevia-derived products have been used in different countries for several years, in Europe they have not been used extensively. FDA approved Stevia for commercialization in 2008 (FDA GRAS 275 and 323) and more recently, on November 2011, the European Commission (EU) has approved steviol glycosides as a new food additive (E 960). Recent green light will probably lead to wide-scale use of Stevia-derived products.10 Stevia rebaudiana may be used as an alternative of synthetic additives in marketed food products.11 So far, little data has been available regarding practical applications in foods.12
Differently from synthetic pharmaceuticals, based upon single chemicals, many phytomedicines exert their beneficial effects through additive or synergistic action of several bioactive compounds. Following research focused on increasing antioxidant consumption in a healthy diet, as well as providing alternatives for decreasing sugar consumption, the aim of this work was to study beverages based on papaya, mango and orange juice, acai, oat beverage and the use of Stevia rebaudiana water extracts as sources of non-caloric sweeteners to formulate, as well as to evaluate antioxidant capacity and synergistic interactions between bioactive compounds in this beverage.
Preparation of orange, mango and papaya juice, oat beverage, açaí and stevia stock solution
Cultivars of papaya (Carica papaya), mango (Mangifera indica), oranges (Citrus sinensis L.), Navel Variety and oat beverage (Santiveri, Lérida, Spain) were purchased from a local supermarket. Papaya, mango and orange juices were extracted after appropriate washing and hygienization of the fruits and the pulp was removed. Açaí (containing 450 mg of açaí berries extract, with 10% of polyphenols) was provided by Nature’s Way Products Inc. (Utah, USA).
Stevia rebaudiana leaves were supplied by company Anagalide, S.A. (Barbastro, Huesca, Spain) and stored at room temperature. A stock solution (8.33%w/v) of Stevia rebaudiana was prepared in order to formulate the beverage. For this purpose, 100mL of bottled water at 100°C were added on the dried leaves (8.33g) and were kept for 30min. An infusion was Vacuum filtered using filter paper (Whatman no 1) and filtrate obtained was stored for the duration of the experiment at –40°C.
Preparation of beverages
Beverages were prepared by mixing 32.5%, 10%, and 7.5% (v/v) of papaya, mango and orange juices, respectively, with the pulp removed, 20% (v/v) of oat beverage, and 30% (v/v) of water (0% Stevia) or the different Stevia leaves infusion (1.25 and 2.5%w/v). Finally, açaí (1%w/v) was added to the beverage. Solid ingredients were placed in water in the weight proportions indicated. The beverage was prepared just before use. Each sample was prepared in triplicate. The maximum Stevia concentration (2.5%) was selected taking into account the sucrose concentration of commercial fruit based beverages and the sweetness equivalence Stevia/sucrose.13
Chemicals and reagents
Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), as a standard substance (2mM) to measure TEAC, 2,2´-azobis(2-methylpropionamidina)dihydrochloride (ABTS), fluorescein sodium salt, 2,2´-azobis(2-amidinopropane)dihydrochloride (AAPH), disodium metabisulfite, Folin-Ciocalteu (ammonium molibdotugstat) reagent, rebaudioside A, stevioside hydrate and steviol hydrate were purchased from Sigma (Steinheim, Germany). Gallic acid 1-hydrate in distilled water, as a standard (10mg/mL) for phenolic compounds, was purchased from UCB (Brussels, Germany). Oxalic acid, acetic acid, chlorhidric acid, acetone, sodium acetate and potassium persulphate (K2S2O8) were purchased from Panreac (Barcelona, Spain). Di-sodium hydrogen phosphate (anhydrous) (Na2HPO4) and potassium di-hydrogen phosphate (KH2PO4) were obtained from Scharlau (Barcelona, Spain). Ethanol, methanol, acetonitrile, hexane, sodium carbonate anhydrous (Na2CO3), trichloroacetic acid and sodium sulphate proceeded from Baker (Deventer, The Netherlands) While Rebaudioside C and rebaudioside F from Wako (Osaka, Japan). L (+)-ascorbic was obtained from Merck (Darmstadt, Germany).
Polarographic determination of ascorbic acid
Beverage (5mL) was diluted to 25mL with an extraction solution (oxalic acid 1%,w/v, trichloroacetic acid 2%,w/v, sodium sulphate 1%,w/v). After vigorous shaking, the solution was filtered through a folded filter (Whatman no 1). Oxalic acid (9.5mL) 1% (w/v) and 2mL of acetic acid/ sodium acetate 2 M buffer (pH=4.8) were added to an aliquot of 0.5mL of filtrate and the solution was transferred to the polarographic cell. A Metrohm 746VA Trace Analyzer (Herisau, Switzerland) equipped with a Metrohm 747VA stand was used for polarographic determination. Working electrode was a Metrohm multi-mode electrode operated in the dropping mercury mode. A platinum wire counter electrode and a saturated calomel reference electrode were used. The following instrumental conditions were applied: DP50, mode DME, drop size 2, drop time 1s, scan rate 10mV/s, initial potential -0.10V. Determinations were carried out by using the peak heights and standard additions method.4
Total carotenoids
An aliquot of sample (2mL) was homogenized with 5mL of extracting solvent (hexane/acetone/ethanol, 50:25:25, v/v) and centrifuged for 5min at 4000 rpm at 5°C. The top layer of hexane containing the color was recovered and transferred to a 25-mL volumetric flask. The volume of recovered hexane was then adjusted to 25mL with hexane. Total carotenoid determination was carried out on an aliquot of the hexane extract by measuring absorbance at 450nm.4 Total carotenoids were calculated using an extinction coefficient of β-carotene, E1%=2505.
Phenolic compounds
Total phenols were determined according to the method reported by Georgé et al.14 with some modifications. Briefly, 10mL of sample was homogenized with 50mL of a mixture of acetone/water (7/3v/v) for 30min. Mixture supernatants were then recovered by filtration (Whatman no 2, England) and constituted the raw extracts (REs). REs (2mL) were settled on an Oasis cartridge (Waters). Interfering water-soluble components (reducing sugars, ascorbic acid) were recovered with 2x2mL of distillated water. The recovered volume of the washing extract (WE) was carefully measured. In order to eliminate vitamin C, heating was carried out on the washing extract (3mL) for 2h at 85°C and led to the heated washing extract (HWE). All extracts (RE, WE, and HWE) were submitted to Folin-Ciocalteu method, adapted, and optimized4: Sodium carbonate solution (3mL) 2% (w/v) and 100μL of Folin–Ciocalteau reagent were added to an aliquot of 100μL of sample. The mixture was incubated for 1 h at room temperature. Absorbance was measured at 765nm.
Total anthocyanins
Total anthocyanins were determined using a modified method of Mazza et al.15 A 10-fold diluted sample of 100μL was mixed with 1700μL of distilled water and 200µL of 5% (v/v) HCl. The sample was holding at room temperature for 20min before measuring the absorbance at 520nm in a 10mm cuvette. Calculations of total anthocyanins were based on cyanidin-3-glucoside (molar absorptivity 25,740). All spectrophotometric analyses were performed using a UV–visible spectrophotometer Lambda 20 (Perkin-Elmer, Überlingen, Germany).
Total antioxidant capacity
Trolox Equivalent Antioxidant Capacity (TEAC) assay The Trolox Equivalent Antioxidant Capacity (TEAC) test was determined according to the method reported by Barba et al.4 based on the capacity of antioxidants to inhibit the radical cation 2,2-azino-bis(3-ethylbenzothiazoline6-sulphonate) (ABTS), which has a characteristic long-wavelength absorption spectrum, showing a maximal peak at 734nm. The ABTS radical cation is formed by the interaction of ABTS (7mm) with K2S2O8 (2.45mm).
Oxygen radical absorbance capacity (ORAC) assay
The oxygen radical absorbance capacity (ORAC) assay used, with fluorescein as the “fluorescent probe”, was that described by Barba et al.4 Automated ORAC assay was carried out on a Wallac 1420 VICTOR2 multilabel counter (Perkin-Elmer, USA) with fluorescence filters, for an excitation wavelength of 485nm and an emission wavelength of 535nm. Measurements were made in plates with 96 white flat bottom wells (Sero-Wel, BibbySterilin Ltd., Stone, UK). Reaction was performed at 37°C, as the reaction was started by thermal decomposition of AAPH in 75mm phosphate buffer (pH 7.0).
PH was determined in a Crison GLP 21 pH-meter (Barcelona, Spain) equipped with a temperature compensation sensor at 20°C. Brix was determined with an Atago RX-1000 digital refractometer (Atago Company Ltd., Tokyo, Japan). To measure the turbidity index (TI), a sample was centrifuged (618×g, 10min, 20°C), supernatant was taken, and absorbance at 660nm was measured.16 To determine the browning index (BI), a sample was centrifuged (824×g, 20min, 18°C), and the supernatant was taken and diluted with ethanol (1:1, v/v). The mixture was filtered with Whatman no 42 filters and absorbance of the filtrate was measured at 420nm.17 Hydroxymethylfurfural (HMF) content was measured using the method described by International Federation of Fruit Juice Producers (IFFJP) (1984). The color analysis was performed using a Hunter Labscan II spectrophotometric colorimeter (Hunter Associates Laboratory Inc., Reston,VA., U.S.A.) controlled by a computer that calculates color ordinates from the reflectance spectrum. Results were expressed in accordance with the Commission International d′Eclairage LAB (CIELAB) system with reference to illuminant D65 and with a visual angle of 10°. Three consecutive measurements of each sample were taken. L* (lightness [0=black, 100=white]), a* (–a*=greenness, +a*=redness) and b* (–b*=blueness, +b*=yellowness).
The method of Joint FAO/WHO Expert Committee on Food Additives (JECFA) (JECFA, 2010) withVArious modifications was used. Samples were filtered through a Sep-Pak® cartridge (a reverse-phase C-18 cartridge; Millipore, MA, USA) which retains steviol glycosides. The cartridges were previously activated with 10mL of methanol (MeOH) and 10mL of water. Every 10mL of sample was eluted with 2mL of MeOH, and all methanolic fractions were collected, filtered through a 0.45 µm membrane filter Millex-HV13 (Millipore) and then analyzed by liquid chromatography. Kromasil 100 C18 precolumn (guard column) (5µm, 150 x 4.6mm); Kromasil 100 C18 column (5µm, 150 x 4.6mm) (Scharlab, Barcelona, Spain). The mobile phase consisted of two solvents: Solvent A, acetonitrile and Solvent B, 10mmol/L sodium phosphate buffer (pH=2.6) (32:68v/v). Steviol glycosides were eluted under 1mL/min flow rate and temperature was set at 40°C. Chromatograms were recorded at 210nm. Identification of steviol glycosides was obtained by using authentic standards and by comparing the retention times, while quantification was performed by external calibration with standards.
The interaction factor (IF), which provides an explanation for the mode of interaction, was determined, according to Eq. (1)18
(1)
Where,
AM= measured activity of a mixture of samples, and AT= theoretically calculated mixture activity (based on the dose response of single components at various concentrations). IF Value<1 indicates synergistic interaction; IF>1 indicates antagonism; IF≈1 indicates additional interactions.
All determinations were performed in triplicate. Normality and homoscedasticity (variance homogeneity) were assayed as premises prior to parametric statistical tests, using a Shapiro-Wilk test and a Levene test, respectively, as described by Granato et al.19 When Variances were heterogeneous, dependent Variables were transformed by the Box-Cox transformation. An analysis of Variance (ANOVA) was applied in order to verify whether there were significant differences in the parameters studied in relation to the sample analyzed, and to ascertain possible interactions between factors (differences at p<0.05 were considered significant). Where there were differences, an LSD test was applied to indicate the samples in which differences were observed. A multiple regression analysis was performed to study the influence of bioactive compounds to antioxidant capacity (results are shown in the significant cases, p<0.05). Finally, a study was conducted with the aim of determining whether there were correlations between a pair of Variables (Pearson´s test). All statistical analyses were performed using Statgraphics® Centurion XVI (Statpoint Technologies Inc., USA).
Physicochemical and nutritional characterization of single components
Advantages of fruit mixtures are widely known, such as organoleptic improvements (aromas and flavors combination) and the synergy effects between their nutritional components. In the present study, nutritional and physicochemical characterization of papaya, mango and orange juices as well as oat beverage, Stevia rebaudiana extracts and açaí capsules were conducted in order to determine potential interactions among the antioxidant compounds when the fruits were combined (Table 1). Mango and orange juices had pH Values of 3.53 and 3.90, respectively, while papaya juice (5.40) Stevia (6.97) and oat beverage (7.52) had higher pH Values. pH is related to the stability of bioactive compounds in fruit-derived products. Thus, the combination of different ingredients with different pH can avoid oxidation reactions and microbial deterioration of food by decreasing pH Values.20 Total soluble solids content of fruits Varied from 10.70 to 14.30 °Brix; the products are thus indicated for different consumers, submitted to diets with different calories. These contents were similar to those found by other authors in mango and papaya purées.21,22
|
Orange |
Mango |
Papaya |
Oat |
Stevia infusion 8.33% (w/v) |
Açaí |
Bioactive compounds and antioxidant capacity |
||||||
Ascorbic acid (mg/100 mL) |
31.85±0.28 |
22.99±0.60 |
57.81±0.78 |
- |
- |
- |
Total carotenoids (µg/100 mL) |
421.90±31.82 |
639.00±36.77 |
708.75±46.57 |
369.60±28.28 |
- |
97.33±3.50 |
TPC (mg GAE/L) |
132.84±3.59 |
84.90±2.26 |
105.23±0.47 |
79.45±1.48 |
1216.30±12.30 |
10.55±0.07 |
TA (mg/L) |
- |
251.86±1.62 |
- |
- |
0.22±0.03 |
280.45±13.46 |
TEAC (mM TE) |
27.63±4.28 |
4.75±0.75 |
7.30±0.78 |
2.16±0.06 |
61.30±0.28 |
18.98±0.05 |
ORAC (mM TE) |
8.22±0.35 |
2.78±0.85 |
6.11±0.47 |
0.93±0.11 |
122.05±0.78 |
18.94±0.33 |
Physicochemical properties |
||||||
pH |
3.90±0.04 |
3.53±0.04 |
5.40±0.03 |
7.52±0.01 |
6.97±0.06 |
6.35±0.07 |
ºBrix |
11.90±0.14 |
14.30±0.14 |
10.70±0.14 |
11.75±0.07 |
1.15±0.07 |
- |
HMF (mg/L) |
0.07±0.01 |
0.16±0.02 |
0.14±0.02 |
0.14±0.01 |
- |
- |
Turbidity index |
0.20±0.01 |
2.48±0.02 |
0.33±0.01 |
0.25±0.01 |
2.44±0.01 |
1.15±0.01 |
Browning index |
0.09±0.01 |
0.10±0.01 |
0.08±0.01 |
0.05±0.01 |
2.77±0.03 |
3.01±0.02 |
Lightness (L*) |
48.36±0.05 |
36.47±0.03 |
52.08±0.02 |
68.55±0.05 |
22.53±0.03 |
31.66±0.50 |
Redness (a*) |
3.72±0.04 |
23.46±0.04 |
25.79±0.01 |
-1.94±0.04 |
12.50±0.02 |
8.82±0.13 |
Blueness (b*) |
50.36±0.05 |
46.79±0.04 |
61.74±0.02 |
6.38±0.02 |
20.36±0.04 |
18.40±0.02 |
Steviol glycosides |
||||||
Rebaudioside A (mg/100 mL) |
- |
- |
- |
- |
1061.24±40.85 |
- |
Rebaudioside C (mg/100 mL) |
- |
- |
- |
- |
222.08±7.46 |
- |
Stevioside (mg/100 mL) |
- |
- |
- |
- |
1881.70±1.47 |
- |
Rebaudioside F (mg/100 mL) |
- |
- |
- |
- |
56.04±3.51 |
- |
Table 1 Bioactive compounds, total antioxidant capacity (TAC), physicochemical properties and steviol glycosides profile of the different ingredients used in the formulation of the samples
Per mg/g/kg product-Non detectable. TPC, total phenolic compounds; GAE, Gallic acid equivalent; TA, Total anthocyanins; TEAC, Trolox equivalent antioxidant capacity; ORAC, oxygen radical antioxidant capacity.
As can be seen in Table 1, the selected fruits constitute a good source of ascorbic acid (22.99-57.81mg/100mL), carotenoids (421.90-708.75µg/100mL), phenolic compounds (84.90-132.84 mg gallic acid equivalents (GAE)/L) and antioxidant capacity (TEAC (4.75-27.63mm TE) and ORAC (2.78-8.22mm TE)). Fruit juices were mixed with oat beverage, which is a good source of proteins from vegetal origin, and showed also a high content in carotenoids (369.60±28.28µg/100mL). In addition, Stevia rebaudiana extract can be used as a natural sweetener, due to its high content in steviol glycosides (rebaudioside A (1061.24±40.85mg/L), rebaudioside C (222.08±7.46mg/L), stevioside (1881.70±1.47mg/L), and rebaudioside F (56.04±3.51mg/L)) with sweeting properties, and can also be a useful tool in order to increase phenolic consumption (1216.30±12.30 mg/100mL) and total antioxidant capacity of food products (TEAC (61.30±0.28mm TE) and ORAC (122.05±0.78mm TE)). Moreover, açaí fruit is another exotic fruit which has attracted researcher’s interest as to its high nutritional properties.23 for this reason; it was selected as an ingredient in the formulation of highly nutritional antioxidant beverages. As can be seen in Table 1, açaí capsules had a high content in anthocyanins and total antioxidant capacity.
Phenolic compounds, total anthocyanins, ascorbic acid and total carotenoids in the formulated beverages
Total phenolic content (TPC) in the beverage based on exotic fruits (mango juice, papaya juice and açaí) mixed with orange juice, oat and without Stevia was of 66.4 mg gallic acid equivalents (GAE)/L. As can be seen in Table 2, TPC was significantly (p<0.05) higher when Stevia at 1.25 (~3-fold) and 2.5% (~4-fold) was added to the formulation. These results were in concordance to those obtained by different authors who reported Stevia rebaudiana as an excellent source of phenolic compounds.24–26
|
% SR |
||
Parameter |
0 |
1.25 |
2.5 |
Bioactive compounds and antioxidant capacity |
|||
Ascorbic acid (mg/100 mL) |
24.8±0.2a |
24.9±0.2a |
24.6±0.2a |
Total carotenoids (µg/100 mL) |
436.6±17.6a |
399.2±35.3a |
424.2±35.3a |
TPC (mg GAE/L) |
230.8±10.9a |
2353.8±16.1b |
4715.4±15.4c |
TA (mg/L) |
22.0±1.4a |
27.8±1.4b |
29.7±0.3c |
TEAC (mM TE/L) |
6.4±0.3a |
20.3±2.2b |
30.4±0.7c |
ORAC (mM TE/L) |
5.1±0.1a |
23.5±0.1b |
36.1±0.1c |
Physicochemical properties |
|||
pH |
4.38±0.20a |
4.50±0.10a |
4.49±0.10a |
ºBrix |
7.70±0.14a |
8.70±0.14b |
9.70±0.20c |
HMF (mg/L) |
0.057±0.003a |
0.099±0.004b |
0.224±0.031c |
Turbidity index |
2.430±0.006a |
2.620±0.005b |
2.798±0.005c |
Browning index |
0.080±0.003a |
1.140±0.004b |
2.416±0.005c |
Lightness (L*) |
59.0±0.1a |
40.7±0.2b |
33.5±0.1c |
Redness (a*) |
11.9±0.1a |
8.5±0.1b |
9.2±0.2b |
Blueness (b*) |
38.5±0.1a |
31.8±0.2b |
30.8±0.1c |
Steviol glycosides (mg/100 mL) |
|||
Rebaudioside A |
- |
171.5±1.5 |
286.9±8.4 |
Rebaudoside C |
- |
30.1±0.6 |
63.6±0.1 |
Stevioside |
- |
363.8±2.6 |
637.5±3.0 |
Rebaudioside F |
- |
7.5±0.1 |
14.6±0.1 |
Table 2 Bioactive compounds, total antioxidant capacity, physicochemical properties and Steviol glycosides of three different beverages mixture of exotic fruit juices and oat beverage, sweetened with 0%, 1.25% and 2.5% Stevia
a–c Different letters in the same row indicate a significant difference in function of the samples analyzed (p<0.05). TPC: total phenolic compounds. GAE, Gallic acid equivalent; TA, Total anthocyanins; TEAC, Trolox equivalent antioxidant capacity; ORAC, oxygen radical antioxidant capacity; HMF, Hydroxy methyl furfural.
Total anthocyanins (TA) in the beverage without Stevia were found in a concentration of 22.0±1.4mg cyanidin-3-glucoside/L. However, this Value was 1.3- and 1.4-fold higher when Stevia at 1.25% and 2.5% Stevia (w/v) was used as a sweetener, respectively (Table 2).Muanda et al25 reported Values of 0.35 mg total anthocyanins/g dry matter when they studied the chemical composition of water extracts from Stevia rebaudiana Bertoni and de Rosso et al.6 obtained Values of total anthocyanins ranging from 282 to 303 mg/100 g in açaí. The proposed beverages can be considered an excellent source of total anthocyanins, mainly due to the presence of açaí in its composition. A significant correlation between anthocyanins and total phenolic compounds measured by Folin-Ciocalteu (p=0.0073) was found when the Pearson test was studied for the different Stevia concentrations.
Ascorbic acid concentration in the beverage without Stevia was 24.8±0.2mg/100mL (Table 2). These results were in close agreement with the Values obtained by other authors in papaya, mango and orange.27,28 Non-significant modifications of ascorbic acid content resulting from the presence of Stevia water extracts (1.25% and 2.5% (w/v)) were found.
Total carotenoids content was of 436.6±17.6µg/100mL in the sample without Stevia and these Values did not increase with the addition of Stevia to the beverage. Experimental results did not show any presence of carotenoids in Stevia-derived products. These results were in accord to those of Muanda et al.25 when they studied the chemical composition SR water extracts.
Antioxidant capacity
The beverage without Stevia added exhibited a more elevated antioxidant activity (p<0.05) when measured with TEAC method (6.4±0.3mm TE) compared to Values obtained with ORAC assay (5.1±0.1mm TE) (Table 2). However, when Stevia was added, antioxidant capacity was higher using ORAC method. The different increase in antioxidant Values of Stevia-sweetened samples measured with both TEAC and ORAC methods can be explained considering that the two tests, apart from differing for the reactive species, are performed at different reaction phases. This consideration suggests that the two tests furnished a diverse kind of information as they emphasized differently the antioxidant capacity of hydrophilic and hydrophobic antioxidants compounds. Results indicated that in Stevia-sweetened beverage, hydrophobic constituents contributed to antioxidant capacity in higher amounts than water soluble antioxidants.
Independently of the method employed, antioxidant capacity of beverages sweetened with Stevia at 1.25 and 2.5%w/v, was higher than that obtained in beverages without Stevia (Table 2). This was more evident when ORAC method was used. ORAC Values were 5 and 7 times higher for beverages with 1.25% and 2.5% (w/v) compared to the beverage without Stevia while TEAC Values were about 3 and 5 times greater for samples with 1.25 and 2.5% (w/v) Stevia, respectively. Therefore, the addition of Stevia contributed to a considerable increase of the antioxidant activity in both of the beverage types.
A significant correlation between TEAC and ORAC Values (p<0.05) was found. These results were in accordance with those found by other authors in different liquid food matrices.29 Furthermore, Pearson´s test showed that antioxidant activity determined by TEAC and ORAC was significantly correlated (p<0.05) with total phenolic compounds and total anthocyanins. This was also observed by Granato et al.30 when they analyzed Various juices from different botanical origins.
Physicochemical properties
Results obtained for physicochemical properties of the beverages analyzed in the present study are shown in Table 2. The Values of pH and ºBrix in the beverage without Stevia were 4.38±0.20, and 7.70±0.14, respectively. Non-significant changes were found in pH Values when Stevia was added as a sweetener, while a significant increase was found in ºBrix. The Values of hydroxymethylfurfural (HMF), turbidity index and browning index of the samples without Stevia added were 0.057 mg/L, 2.430, and 0.080, respectively. As can be observed in Table 2, a significant increase was obtained in HMF content, turbidity and browning of the samples when Stevia percentage was higher in comparison to the beverage without Stevia. However, parameters which define the colour of the samples (L*, lightness; a*, redness; and b*, blueness) decreased significantly when increasing Stevia percentage compared to sample without Stevia added (L*= 59.0; a*= 11.9; b*= 38.5).
Steviol glycosides
Results obtained for steviol glycosides of the beverages analyzed in the present study are shown in Table 2. Four different steviol glycosides (rebaudioside A (reb A), rebaudioside C (reb C), rebaudioside F (reb C), and stevioside (ste) were detected and quantified (Table 2, Figure 1) in samples containing Stevia. Stevioside was the predominant steviol glycoside identified in the beverages with Stevia at 1.25% (363.8mg/100mL) and 2.5% (637.5mg/100mL), while the lower Values were found for rebaudioside F. As can be expected, the ANOVA analysis showed a significant increase (p<0.05) in reb A, reb C, reb F and ste when Stevia percentage used in the formulation of the beverages was increased, enhancing the sweetening properties of the beverages. In previous studies, Carbonell-Capella et al.31 demonstrated that steviol glycosides standards showed antioxidant capacity using the ORAC method, so the presence of these compounds in the beverages could explain in part the increase of the antioxidant capacity measured with ORAC method.
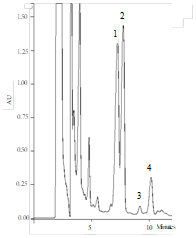
Figure 1 Chromatogram HPLC analysis of steviol glycosides 1: Rebaudioside A; 2: Stevioside hydrate; 3: Rebaudioside F; 4: Rebaudioside C in a beverage mixture of exotic fruit juice and sweetened with Stevia rebaudiana (SR) Bertoni at 2.5% (w/v).
Interaction assay
In order to investigate in profundity the antioxidant capacity of the bioactive compounds found in the orange, mango and papaya juice combined with oat beverage and açaí and sweetened with different concentrations of Stevia water extracts, the interaction factor (IF) was determined (Table 3). This assay is a simple way to explain the mode of interaction and may be used to make a preliminary assessment of the types of interactions between the examined extracts or chemical compounds. Antioxidant activity of beverages and theoretically calculated mixture activity (based on the dose response of single components at various concentrations) was calculated.
Stevia rebaudiana |
Activity |
AM |
AT |
IF |
0% |
TEAC |
6.44 |
5.54 |
1.16 |
|
ORAC |
5.05 |
3.25 |
1.55 |
1.25% |
TEAC |
20.30 |
14.73 |
1.38 |
|
ORAC |
23.46 |
21.56 |
1.09 |
2.5% |
TEAC |
30.41 |
23.93 |
1.27 |
|
ORAC |
36.09 |
39.87 |
0.91 |
Table 3 Comparison of interaction factors (IF) in the beverages sweetened with Stevia rebaudiana (0, 1.25 and 2.5%)
AM, Measured activity; AT, Theoretical calculated activity; TEAC, Trolox equivalent antioxidant capacity; ORAC, Oxygen radical antioxidant capacity.
When examining the antioxidant capacity using the TEAC method, antiradical scavengers included in the fruit juices, oat beverage and açaí acted synergistically in the beverages without stevia added. In the case of 1.25% and 2.5% stevia beverages, the same kind of interaction was observed. Unlike in the case of synthetic pharmaceuticals based on the activity of single active compounds, numerous phytochemicals act in a beneficial manner via addition of synergistic activity in target sites connected to physiological processes. Synergistic interactions observed when TEAC assay is employed in the beverage sweetened with 2.5% stevia suggest an improved solubility or stability of the antioxidant compounds and therefore, the combined antioxidant capacity of the mixture measured by TEAC method is potentiated. These results are in line with those reported by Gawlik-Dziki32 who observed synergistic interactions for constituents within the total extracts of a single plant-derived product, as well as between different plant products in a formulation, obtaining that the whole or partially purified extract of a fruit and vegetables offers advantages over a single isolated ingredient.
The antioxidant capacity measured using the ORAC method also revealed synergistic interactions between the single components of the beverages without stevia. However, the addition of 1.25% and 2.5% of stevia to the beverages indicated additional interactions (≈1) between the individual components of the beverages. Unlike TEAC Values, antioxidant activity measured with ORAC method when stevia is included in the formulation was similar to that predicted, revealing that the presence of stevia water extract may be engaged in shaping the potential antioxidant activity of the studied beverages. Several studies conducted on food products fortified with phenolic-rich ingredients such as onion skin,32 quinoa leaves33 and parsley leaves34 show that part of the antioxidant activity may be masked as a result of interactions between selected phenolic compounds with other food components. Nevertheless, despite these possible interactions, the addition of phenolic rich ingredients results in the promotion of the total antioxidant capacity of foods.35–38
The beverage based on exotic fruits (mango juice, papaya juice and açaí) mixed with orange juice, oat and sweetened with Stevia rebaudiana water extracts at 2.5% (w/v) was found to contain the highest amount of total phenolic compounds, almost 4-fold higher than the sample without stevia, and consequently presented the highest antioxidant capacity measured both with ORAC and TEAC method. From these results, it can be concluded that the use of Stevia rebaudiana as a natural non-caloric sweetener can also be a good source of bioactive compounds. Synergistic interactions observed for phytochemicals and steviol glycosides in the complex food beverages when TEAC method was used suggest an improved solubility, stability and/or different mechanisms of action of antioxidant compounds and hence, the combined antioxidant capacity of the mixture measured by TEAC assay is potentiated in the complex food matrix.
This research project was supported by the Spanish ministry of Science and Technology and European Regional Development Funds (AGL2010-22206-C02-01). J.M.CC. holds an award from the Spanish ministry of Education (AP2010-2546).
None.
None.

©2019 Carbonell-Capella, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.