MOJ
eISSN: 2381-182X


Research Article Volume 5 Issue 4
1School of Food Science and Technology, Jiangnan University, China
2Department of Marine Food and Biotechnology, Massawa College of Marine Science and Technology, China
Correspondence: Bereket Abraha, School of Food science and Technology, Jiangnan University, Wuxi 214122, China, Tel +86-186-1667226, Fax +86-510-85809610
Received: October 09, 2017 | Published: December 14, 2017
Citation: Abraha B, Mahmmud A, Samuel M, et al. Production of fish protein hydrolysate from silver catfish (Arius thalassinus). MOJ Food Process Technol. 2017;5(4):328-335. DOI: 10.15406/mojfpt.2017.05.00132
Fish protein hydrolysates are broken-down products of enzymatic conversion of fish proteins into smaller peptides, which normally include 2-20amino acids. In recent years, fish protein hydrolysates have attracted much attention of food technologists due to the availability of ample quantities of raw material for the process and presence of high protein content with good amino acid balance, minerals as well as vitamins. In the present study, fish protein hydrolysate was produced for human consumption using papain enzyme from plant origin optimally achieved at neutral pH.
Catfish (Arius thalassinus) a low-value fish from Eritrean Red Sea waters was used as substrate. The process consisted of dressing, separation of flesh by boiling and hand picking, washing the flesh, separating the dark flesh from bones, enzymatic digestion, deactivation of enzyme, drying and powdering. Under optimal condition, hydrolysis of protein by enzymes yielded products containing coagulable nitrogen, proteases, peptones, sub-peptones and free amino acid depending on the extent of degradation of proteins which is related to the type, concentration of enzyme and condition of hydrolysis (temperature and time). The product was high in protein (80-85%) and ash (5.2%) contents and low in fat (0.5%), and moisture content (6.8%). Solubility of the Fish protein hydrolysate was investigated using water and found soluble in water, which makes it an alternative protein supplement for undernourished population. The product was tested in a beverage form by taste panelists for its acceptability and was acceptable to 80% by the respondents.
Keywords: catfish (arius thalassinus), proximate composition, fish protein hydrolysate, papain
Fish are highly nutritious food items. In most countries, fish is one of the significant sources of animal protein and essential nutrients for the maintenance of health and protection from different types of diseases, such as blood pressure, cancer and heart stroke.1 Sea food products reduce vulnerability to hunger by providing a complementary food source when other food sources, such as agriculture are at a seasonal low.2 Foran et al.3 stated in the findings, fish is a highly proteinaceous food consumed by a larger percentage of population in the world because of its availability and palatability. Today there is an ever increasing know-how about healthy food and fish is finding more acceptances because of its special biochemical and nutritional qualities.4
In most of the developing countries, or the so called emerging countries, Eritrea being one of them, there have not been such significant advances in health of the population. The result is a wide spread malnutrition, particularly protein malnutrition for the vulnerable population especially children, pregnant mothers etc. Likewise, the exploration of population in these countries, global climate changes have greatly reduced the proportion of agricultural land and besides lack of skilled manpower and inputs in fisheries sector have contributed to low outputs of fish capture, that could provide vitally needed animal proteins.5 Abraha et al.5 and6 reported, Eritrea's coastal waters are among the most productive fishing grounds in the Red Sea. The existing fish population of large and small pelagic fishes includes: tuna, queen fish, catfish, mackerel, barracuda, shark, grouper, anchovies, sardine etc. The Maximum Sustainable Yield of marine fish resource of Eritrean Red Sea is estimated 80,000 metric tons per year. Sliver catfish, Arius thalassinus, are abundantly found in the Dahlak Archipelago Islands of Eritrean Read Sea waters. Catfish are among the low values fishes which have very low consumption rate compared to coral reef fishes. Moreover, this species has poor acceptance in the consumer market.5,7 These are underutilized resources of fish, that man could turn them to sources of animal protein in an enormous quantity at low cost.2 What is needed is to develop a method of using fish as high quality protein supplement to upgrade lower quality vegetable proteins, which are presently the main source of this important nutrient. The product produced from fish must be easy to store, distribute and retail at ambient temperatures to enable to reach the remote areas of the country and yet retaining its high nutritional value. The product produced must be available in a form to be introduced in to a wide variety of traditional meals to ensure that it can enhance to increase nutritional content diets of the people, moreover the targeted consumers. Fish Protein Hydrolysate (FPH) is the best example of these, prepared by concentrating fish proteins. Catfish hydrolysates powder has high protein content due to the solubility of protein during hydrolysis; removal of insoluble and undigested non protein substances; and partial removal of lipid after hydrolysis.8
Preparation of fish protein hydrolysate (FPH) using low value fish and fish wastes to achieve better utilization of fish, have received the attention of researchers for the last five to six decades.9,10 Patents on preparation of peptones and amino acids were taken in early 1950’s in Japan and U.S. Work on fish protein hydrolysate was initiated in India in early 1960’s at the central food technological research station.11
In addition to over exploitation and wastage of protein rich food, more than 50% of fish is considered as inedible or processing wastes comprising of head, skin, bone, scales, fins and viscera which are not utilized for food and mostly used as animal feed.4 In recent years, strict environmental regulations are no longer allowing processing companies to discard wastes. Therefore there is an urgent need to eliminate by-catch by designing efficient gears, control post-harvest fish losses and develop alternative methods for better and complete utilization of fish.2
FPH are prepared by treating fish or fish waste with proteases, the soluble fraction is concentrated to get powdered protein hydrolysates which has better functional and nutritive properties as well as high solubility in water compared to fish protein concentrate.12,13 By using different fish species, enzymes and digestion conditions, a wide range of hydrolysates can be developed.4 The enzymes can be of plant (papain, bromelin, ficin), animal (pepsin, trypsin etc.), or microbial (pronase, alcalase, etc.) origin.2 By careful control of hydrolysis, it is possible to modify the functional properties such as water holding capacity, emulsifying and foaming ability of proteins. The degree of hydrolysis is important to optimize the process parameters to get a high quality product.4,14
Protein hydrolysates are generally used for modification of functional properties of foods and use of low cost raw material content of high quality proteins. The high solubility property of the hydrolysates also adds to their features of being an ideal protein supplement to vulnerable population of developing and developed countries.2,15 Production of fish protein hydrolysate could be the best option to upgrade low value fish such as silver catfish, thereby increasing their consumer acceptability and ultimately improve their consumption rate. This study was, therefore, undertaken to produce and evaluate acceptability of fish protein hydrolysate prepared from underutilized low value silver catfish from Eritrean Red Sea. Pre-school children and pregnant/nursing mothers are the most beneficiaries of the high nutritional values of this product.
Study location
The study was conducted at the Marine Biotechnology laboratory, in Massawa College of Marine Science and Technology, Eritrea.
Materials used
The experimental fish was silver catfish (Arius thalassinus) ,16 caught around Dahlak Archipelago Islands of Eritrean Red Sea waters by gill net and kept in fish boxes using ice and transported to the laboratory of Massawa College of Marine Science and Technology. Rough balance of 5Kg capacity/0.25g and fine balance of 300g capacity/0.1g were used for weighing enzyme, flesh and dried powder products. A thermostatically controlled vacuum oven drier type VP-3, No= 5505 having 40- 220°C capacity, was used for drying the product. A digital thermometer with a range between -50 -300°C (LCD portable multi-system thermometer; No. 201211253516 Hutaib Enterprise, India) was used for measuring temperature at various stages. Boiling bath with serial No= 56638, made up of nickel electron LTD with working capacity of 1-100°C was used for the temperature controlled liquefaction or hydrolysis process. Dishes and white cloth mesh were used for boiling process and squeezing process.
Methodology
Preparation of protein hydrolysate: The hydrolysis was carried out as describe by.14 A 5 Kg of silver catfish (Arius thalassinus) preserved in ice was used in preparation of the protein hydrolysate. Fish sample was organoleptically of sound quality having uniform appearance, maturity and size. It was handpicked from the lot and used for the study. The procedure followed for the preparation of FPH is given in the flow diagram (Figure 1).
Fresh silver catfish (Arius thalassinus) |
↓ |
Weighing |
↓ |
Dressing (Removal of fins and viscera |
↓ |
Weighing |
↓ |
Filleting |
↓ |
Boiling 25-30 min at 100 °C |
↓ |
Separation of flesh (Remove bone, dark meat) |
↓ |
Weighing |
↓ |
Washing with hot and cold water 6 times |
↓ |
Pressing or squeezing using white cloth and weighing |
↓ |
Enzyme treatment (2-5%) at standardize temperature (40 and 50 °C), at neutral pH (6.5-6.8) |
↓ |
Heating to 75-85 °C to inactivate enzymes for mints |
↓ |
Drying at 80 °C in vacuum oven drier for 4-5 hours |
↓ |
Powdering of fish Protein Hydrolysate |
↓ |
Weighing, Packing, Storing |
Figure 1 Flow diagram of fish protein hydrolysate
Initially, the fish was weighed, washed with clean water and dressed to remove the waste parts. The dressed fish was then again washed by clean water to remove some of the dirt remains and then cooked and left to cool. Following the cooking and cooling process, the bones and dark meat were separated manually. The separated flesh was then washed repeatedly with cold and hot water, to reduce fat. The flesh was pressed using a white cloth mesh to remove water and was ground in to small pieces. Then, the flesh was mixed with required amount of enzyme and subjected to hydrolysis for a specified time at particular temperature. After hydrolysis the samples were heated at 75-800°C, to inactivate the enzyme and were subjected to oven drying. The dried samples were blended to produce powder and sieved to get a fine powder. Finally the samples were packed in polythene bags and heat sealed.
Measuring the degree of hydrolysis: The degree of hydrolysis with time and temperature was monitored by the method described.17 Degree of hydrolysis of FPH was measured by taking two different temperatures (45 and 500°C) and four different enzyme concentrations (2%, 3%, 4% and 5%) in triplicate samples were analyzed per each enzyme concentration and at different temperatures. The number of samples were 24 batches, each contain 40g. All batches were treated with known concentration of enzyme. Boiling of samples was done sequentially; and rate of hydrolysis were measured at time interval of 3 minutes.
Proximate composition: The proximate analyses on crude protein, crude fat, ash and moisture were carried out in the fresh fish. The analyses were done according the method of Association of Official Analytical Chemistry (AOAC).18 The following parameters were measured: crude protein (Kjeldahl method), ash (muffle furnace), crude fat (Soxhlet method), moisture content (thermostatically controlled forced air oven, 105°C for 8 hours).
Determination of crude protein: Protein content in the sample was estimated using Micro- Kjeldhal method. Estimation was done in Asmara national laboratory and the principle they follow was kjeldhal method, involves digestion, distillation and titration. FPH samples (1g) were taken in the digestion tube, to this anhydrous potassium sulphate was added to increase boiling point, and copper sulphate was added as a catalyst or to speed up the reaction, concentrated sulphuric acid was added as oxidizing agent. The material was left over night to enhance digestion and then the material was digested in the digestion blocks at the temperature of 420°C, this process is called digestion. The sample was distilled by passing steam, ammonia liberated due to addition of alkali and trapped in boric acid. Boric acid in NH3 was titrating against the standardized hydrochloric acid using methyl red indicator. The percent nitrogen is calculated using the titer value. The protein content was obtained by multiplying the percent of nitrogen with the factor 6.25.
Where,
V1 = volume of H2SO4, ml
V2 = volume of blank, ml
W = weigh of sample, g
N = Nitrogen, %
Protein content = N % × 6.25
Determination of crude fat: Crude fat content was determined using Soxhlet solvent extraction method.18 Petroleum ether a non polar solvent was used for the extraction of crude fat from sample. Sample was placed in the thimble porous material. Petroleum ether was placed in distillation flask and heat was given as a result, solvent was vaporized and condensed on the upper part then cooled and drops to the thimble, then the solvent washed out to the fat present on the food. This process was done continuously for a period of one hour until all the fat present was finished. At the end of experiment solvent was allowed to vaporize. The flask was reweighed along with the fat present in the food by subtracting weight of empty distillation flask from the reweighed weight it can be calculate the amount of fat present in the food.
Moisture content: Residual moisture was determined gravimetrically by heating the sample at 105°C for 8 hours in hot air drying oven until a constant weight has achieved. The moisture content was stated as percentage by dry weight of sample.18
Ash content: Ash content of the sample was determined according to the method of Association of Official Analytical Chemistry.18 Ash content was determined by complete igniting of the sample (4-6 gram) in a muffle furnace at a temperature of 550°C for 6 hours and ash was calculated in percentage as:
Sensory evaluation
Test for acceptability: The consumer's acceptability of the product was evaluated by a group of 30 panelists using the nine point Hedonic rating scale according the method described.19 The panelists were randomly selected from the department of Marine Food and Biotechnology and Eri-Fish processing plant. Hedonic scale was in the following sequence: 9= Like extremely, 8= Like very much, 7= Like moderately, 6= Like slightly, 5= Neither like nor dislike, 4=Dislike slightly, 3= Dislike moderately, 2= Dislike very much and 1= Dislike extremely.
Statistical analysis: The statistical analyses were performed using statistical program (SPSS 18.0) for Windows. All analytical determinations were performed in triplicate and the mean values were reported. The result of hydrolysis with reference to variation in enzyme concentration and temperature were statistically analyzed using two-way ANOVA as describe.20 Difference between mean were reported at a significant level, p≤0.05. Descriptive analyses were also used in organoleptic analysis for the acceptability.
Proximate composition and weight composition of raw material fish
Size and weight of a fish is very important in the yield of fish protein hydrolysate. The average weight of the samples used in this study was 3.85 to 5kg. The data represented at Table 1 are proximate composition of the raw material used in the present study. The proximate composition of silver cat fish was 18.56% crude protein, 0.54% fat, 79.15% moisture and 1.75% ash content. The results of the present study are in agreement with earlier findings,21 who studied Proximate Composition and Technological Properties of Wild African Catfish Chrysichthy snigrodigitatus (Lacépède1802).
Proximate Composition |
% Wet Basis |
Crude protein |
18.56 |
Fat |
0.54 |
Moisture |
79.15 |
Ash |
1.75 |
Table 1 Proximate composition of raw material fish
The weight composition is the contribution of each part of fish to the total weight expressed as percentage. The cat fish sample used in this study constituted of 43-49% edible flesh. The inedible part such as head, gill and skeleton constituted major part of the waste (Table 2), which is in accordance with the result found by.22 The variation in weight composition due to the size and shape of the fish depends on the skill of filleting. This variation within the species appears to be very significant and can be attributed to the difference in size of fish as bigger fish yield higher edible portions.
Parts |
Sample1 |
Sample 2 |
||
Weight Composition |
% Composition |
Weight Composition |
% Composition |
|
Flesh |
1.400 kg |
49.12 |
1.600 kg |
43.54% |
Head, gill, skeleton |
0.875 kg |
30.72 |
1.350 kg |
36.73% |
Viscera |
0.250 kg |
8.77 |
0.350 kg |
9.53% |
Airbladder |
0.050 kg |
1.75 |
0.060 kg |
1.63% |
Fins |
0.125 kg |
4.38 |
0.150 kg |
4.08% |
Skin |
0.150 kg |
5.26 |
0.165 kg |
4.49% |
Table 2 Weight composition of silver cat fish (Samples 1 and 2)
Production data
The yield of FPH from whole cat fish (Arius thalassinus) was found to be (7% and 6.9%) as shown in (Table 3). The yield of flesh was 49% and 43% (Table 2). In most of fishes the 21 ratio of edible to inedible part is 50:50. In the production of FPH, substantial amount of wastes were generated which needs to be efficiently utilized. Thankamma et al.23 have reported a yield of 10.9% from whole catfish (Arius tachysurus) and 7.8% from jaw fish (Johnius spp.). The yield is comparatively less in the present study (7% and 6.9%). This may be due to variation of weight composition and proximate composition especially moisture and fat content which show wide variation in fish.24
Processing details
Fish flesh preparation: For production of FPH raw flesh/minced meat or cooked flesh can be used. In large scale production it is not economical to cook and separate by hand, therefore use of raw minced meat is practical. In the present experiment the dressed fish (catfish) were cooked, pressed and washed. The cooking and pressing operation resulted in a light color flesh. The yield after cooking from separated flesh was 26.31% in sample 1 and 26.08% in sample 2 respectively. Cooking softened the flesh, resulting in breakage of the muscle tissue and partially reduced fat content of 0.728% and 1.604% in Samples 1 and 2 respectively (Table 3). The process has also facilitated easy separation of bone and dark meat, as well as coagulation of proteins. Similar observations were recorded by Sngiyama et al.25 applied on defatted sardine (Sardinella longiceps) and Normah et al.26 in threadfin bream (Nemipterus japonicas). Further washing followed by manual cloth mesh pressing, played a major role in reducing the fat content in addition to the removal of water soluble protein. This is because fat content in flesh is very sensitive to auto-oxidation, tracer of fat may cause flavor reversion during storage.
Cat Fish |
Product Yield in kg |
||||||
Initial Fish wt. |
Flesh |
After Boiling |
After Squeezing |
After Hydrolysis |
After Drying |
Final Powder |
|
Sample No. 1 |
2.85 |
1.35 |
1 |
0.75 |
0.6 |
0.35 |
0.2 |
Sample No. 2 |
3.68 |
1.6 |
1.28 |
0.96 |
0.768 |
0.448 |
0.256 |
Table 3 Yield of fish protein hydrolysate (kg) from the two cat fish samples
Enzyme concentration: In the present study papain was used at various concentration 2%, 3%, 4% and 5% to standardize with optimum level (Table 4) following the method described by Sen et al.27 Enzymes such as papain, has been widely used for hydrolysis of fish protein compared to acid hydrolysis. It was found that 3% papain at 50°C was optimum for the preparation of FPH, which is supported by Bhaskar et al.28 The results of the study were slightly lower than a study conducted by Wisuthiphaet et al.2 who found 6% at 62.23°C as optimal condition for hydrolyzing of fish protein.
Time Taken for Hydrolysis (min) |
|||||
Percent Concentration of Papain Enzyme added |
|||||
2% |
3% |
4% |
5% |
||
Experiment No. 1 at 50°C |
Replica 1 of 1 |
125 |
70 |
40 |
40 |
Replica 2 of 1 |
131 |
66 |
40 |
34 |
|
Replica 3 of 1 |
101 |
60 |
32 |
34 |
|
Experiment No. 2 at 45°C |
Replica 1 of 2 |
156 |
101 |
53 |
41 |
Replica 2 of 2 |
145 |
100 |
53 |
45 |
|
Replica 3 of 2 |
154 |
105 |
56 |
42 |
|
Table 4 Duration of hydrolysis (min) versus enzyme concentration (%)
Temperature of hydrolysis: On the basis of degree of hydrolysis Sripathy et al.29 suggested that temperature and enzyme concentration play an important role in release of essential amino acid. In the present study, it was found that increasing enzyme concentration and temperature have a little positive impact and gave a bitter taste on the final product, so a standardized of 3% enzyme concentration, at 45- 50°C for one hour duration was optimum (Table 5) & (Figure 2). The criterion for the duration of hydrolysis is the solubilization of protein material which was tested during liquefaction by dissolving the hydrolyzed material in water. This signifies that, in incorporating FPH with beverages, solubility is the important factor with respect to temperature. Complete solubilization was considered as optimum degree of hydrolysis. Wisuthiphaet et al.2 stated in their findings that period of hydrolysis could be decided based on the end use of product.
Statistical analysis: The effect of temperature and enzyme on degree of hydrolysis of myofibrillar protein with papain enzyme is given (Table 5) & (Figure 2). Degree of hydrolysis is affected by time, temperature, enzyme concentration and environmental factors like pH.30 Degree of hydrolysis differed (p value˂0.05) depending on the use of papain and reaction time, myofibrillar protein showed higher rate of hydrolysis when treated with 5% papain. The rate of enzymatic hydrolysis increases as enzyme concentration increases .2 Lower enzymatic hydrolysis 2% takes longer time compared to 5% (Table 4) & (Table 5). At higher temperature (50°C), hydrolysis rate was significantly different from 45°C with respect time and higher enzyme concentration was significantly different from lower concentration 2% with respect to temperature (45 or 50°C). Over all hydrolysis rate at 45°C and 50°C was significantly different (Table 2). Degree of hydrolysis was measured depending on the solubility. According to this study, the readily solubilized protein without any residues shows the degradation of protein to simple form. Study on degree of hydrolysis by Sugiyama et al.25 reported that, the peptide content of myofibrillar protein and its hydrolysate were significantly increased with the increase in time incubation (p value˂0.5) from 85.5 to 299.1mg/g estimating alpha-amino nitrogen content.
Proximate composition: The chemical composition of fish and fish protein hydrolysates has an essential role on human health to provide the essential nutrients in order maintain good health. Generally proximate composition of fish protein hydrolysate varies with type of fish, and enzyme used. In the present study, the proximate analyses of the experimental fish were: crude protein content range (81.8-93.1), moisture content (1.4-8.5), ash content (3.2-8.6) and fat content (0.0-5). The high protein content of fish protein hydrolysates obtained in these findings elucidate its potential use as protein supplements for human nutrition specially to children, pregnant women and immune suppressed people. FPH had low moisture content which is related to high temperature employed during processing and drying which has vital role in extending the shelf-life of the product.31,32 This result is in agreement with Martone et al.33 who prepared highly soluble fish protein hydrolysates with 80% protein from hake (Merluccius hubssi). According to Nair et al.24 proximate compositions of FPH from whole fish of thread fin were found: protein 86.4%, fat 0.1%, ash 7.6% and moisture 3.5%. The FPH from catfish prepared in the present study showed similar results (Table 6) & (Figure 3). Higher fat content of FPH compared to previous studies may be due to incomplete removal of fat. Sincefish fat is rich in poly unsaturated fatty acids and is susceptible to auto oxidation, extreme care is required during defatting.8,33
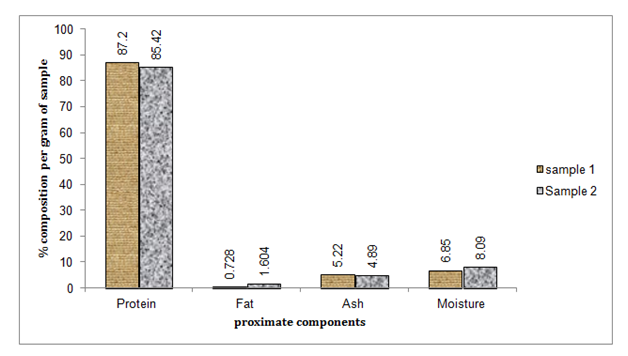
Figure 3 Graphical presentation of proximate composition for fish protein hydrolysate prepared from silver silver catfish.
Temperature |
Percent Concentration of Enzyme |
Mean Time (min) |
Std. Deviation |
50°C |
2% |
119 |
±15.87 |
3% |
65.33 |
±5.03 |
|
4% |
37.33 |
±4.62 |
|
5% |
36 |
±3.46 |
|
Total |
64.42 |
±35.91 |
|
45°C |
2% |
151.67 |
±5.86 |
3% |
102 |
±2.65 |
|
4% |
54 |
±1.73 |
|
5% |
42.67 |
±2.08 |
|
Total |
87.58 |
±45.20 |
Table 5 Statistical result of mean time (min) taken at different enzyme concentration and temperature during hydrolysis
Chemical Constituents of the Fish Protein Hydrolysate |
||||
Protein (%) |
Fat (%) |
Ash (%) |
Moisture (%) |
|
Experiment No. 1 |
87.2 |
0.728 |
5.22 |
6.85 |
Experiment No. 2 |
85.42 |
1.604 |
4.89 |
8.09 |
Table 6 Proximate composition of fish protein hydrolysate
Consumer acceptability of FPH: The overall acceptability of beverage prepared from FPH on a nine point hedonic scale indicated that the product is acceptable to 80 % of the respondents. Comparing “like” to “dislike” percentage responses, FPH was acceptable as shown in the Figure 4. Earlier study reported that fish protein hydrolysate has a problem with bitterness.10 However, in this study the FPH was received best acceptance and very liked by the panelists and the overall acceptability was about 80% out of 30 panelists.
Fish protein hydrolysate is a rich resource of protein (≥85%) or amino acids which can be used for human consumption; it may also be used as constituent of growth media for industrial fermentation process. The market for such products seems to increase as a result of biotechnological research. The important questions in the use of FPH are the variation in molecular weight of different peptides and amino acids sequence and control of product quality. The type of enzyme and processing condition during hydrolysis are critical to achieve a uniform degree of hydrolysis. The therapeutic and health promoting properties of FPH makes them a ‘Neutraceutical’ with promising future. The technology preparation of FPH can be used effectively to upgrade and utilize underutilized fishes such as silver catfish and other varieties. In the preparation it is critical to select a cheap source of enzyme such as papain produced from papaya latex and easily available. Papain is also the enzyme of choice, because its optimal activity is at neutral pH. Hydrolysis from other enzymes will be more expensive. Generally enzyme hydrolysis does not destruct amino acids compare to acids and alkaline hydrolyses.
The temperature, pH and duration of hydrolysis need to be controlled in order to get a uniformly hydrolyzed product. In the present study, FPH was prepared from low value silver catfish at different enzyme concentration and different temperature, standardized with the optimum level of enzyme (3%) and temperature (50°C) with one hour. With further study, the production of fish protein hydrolysates by papain enzyme could be economically suitable and profitable for industrial application, whereby it can alleviate malnutrition and increase consumption rate of low value fish.
The authors are grateful to the Massawa College of Marine Science and Technology for financially supporting the research project. The authors also would like to thank Dr. Zekeria Abdulkerim, Dean of the college, Asst. Professor N.S. Sudhakara, Asst. Professor Pasience Magoha, Mr. Negasi Tsighe (Head department of Marine Food and Biotechnology), laboratory team of Eritrea Standards Institute for their technical support, encouragement and assistance.
The author declares no conflict of interest.

©2017 Abraha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.