MOJ
eISSN: 2381-182X


Research Article Volume 8 Issue 2
Food Engineering and Packaging Department, Food Technology Research Institute, Agricultural Research Centre, Egypt
Correspondence: Food Engineering and Packaging Department, Food Technology Research Institute, Agricultural Research Centre, Egypt, Tel 01112667765
Received: May 26, 2020 | Published: June 30, 2020
Citation: Bakhy EA, Aboulanean HED. Enhancement the antibacterial agent of edible coating and films by incorporating it with green metallic nanoparticles using Luria mobiles leaves. MOJ Food Process Technol . 2020;8(2):80?97 DOI: 10.15406/mojfpt.2020.08.00246
The aim of this study was to produce an edible coating and film from gelatin powder and the incorporating it with action of silver AgNo3 with extracts Luria leaves/glacial acetic acid and the rheological properties, particle size distribution, zeta potential emulsion, and scanning electron microscopy (SEM) of the prepared films were determined. The thickness, tensile strength, elongation, % solubility of produced by edible film with silver AgNo3 with extracts Luria leaves/glacial acetic acid was the highest, followed by that of gelatin powder-based film with extracts Luria leaves/glacial acetic acid. Therefore, the addition of silver AgNo3 with extracts Luria leaves/glacial acetic acid or extracts Luria leaves/glacial acetic acid to gelatin powder films have the potential to provide a safe edible films decreased microbial growth and consequently prolonged the shelf life of strawberry, As well as improved the physio-chemical changes properties of the strawberry .The substances used in this experiment were , film with a 0.25ml , film with b 0.50ml , film with c 0.75 ml , film with d 1.0 ml, film with f 0.25 ml, film with g 0.50 ml, film with h 0.75 ml, film with i 1.0 ml compared film with (e and j) on quality attributes and prolong shelf- life of strawberry were studied during storage at 0.5°C and 90-95% relative humidity (RH) for 12 days from storage . The results observed that was the best treatment in terms of reduction of microbial load followed by treatment (a, b, c and d) followed by treatment (f, g, h and i) until 12days of storage as compared to control (e and j) without nanomaterials additives, during the manufacture of the films. The levels of decrease in chemical and microbial load in the samples from both of the edible films were related good quality strawberry. On the other hand observed that coefficients ( a, b, c and d ) gradually increased with increasing the concentration of antimicrobials action of extract Luria leaves/acetic acid glacial synthetic nanoparticles size 0.25, 0.50, 0.75 and 1.0 the results were as follows 332.3, 407.0, 411.0 and 577.0 respectively as compared control (e), which was higher than treatments 660.2. It was found that the treatments of antimicrobials action of silver nitrate AgNo3 and extract Luria leaves/acetic acid glacial synthetic nanoparticles size (f, g, h, i ) gradually decreased with increasing concentration of 0.25, 0.50, 0.75 and 1.0 and the results were 17.7, 14.50, 12.64 and 5.70 as compared the control (J), which was significantly higher than the coefficients 71.26. It is clear from this that the concentration of silver nitrate AgNo3 and extract Luria leaves in treatments (f, g, h, I and j) was better than other extract luria leaves/acetic acid glacial (a, b, c, d and e).
Keywords: Strawberries, shelf-life, edible coating and films, gelatin, silver nitrate AgNo3, extract luria leaves/acetic acid glacial
The evolution of reliable process for the installation of silver nanoparticles is a remarkable side of influx nanotechnology as the most active area of research. Nanomaterial’s such as sliver particles has been synthesized by different methods, including hard template through using bacteria fungi and plants, which play a significant role in the field of medicinal and biological applications due to its physiochemical properties, for instance the antimicrobial activity of silver nanoparticles toward gram negative bacteria like Escherichia coli.1 Antimicrobial edible films are a fast develops technology that can be used to control the microbiological degradation of perishable food products. Different organic and inorganic active antimicrobial agents can be inserted into the film templates to prevent unfavourable microbial spoilage revolving during storage of packaged fresh food. Although, most films used to preserve food stuff have been produced from synthetic polymers; nevertheless, for environmental reasons, there is an attention focused lately on natural biopolymers such as polysaccharides, proteins and lipids or the combination of these components for the preparation of food packaging films. These films are usually loaded with antimicrobial agents, who come in contact with food stuff, act upon food born microorganisms and inhibit their growth.2 Packaging material having antimicrobial activity limits or prevents microbial growth by reducing growth rate or extending lag phase of microorganisms. Hence, it leads to an extension of the shelf life and the improved safety of the product. Because of the increase in consumer demand for minimally processed, preservative-free products, the preservative agents must be applied to packaging in such a way that only low levels of preservatives come into contact with the food or natural preservative materials should be used. In order to meet this demand, the film or coating technique is considered to be more effective for applications.3 The natural antimicrobial agent incorporation into a polymeric material for the development of antimicrobial food packaging materials is a new subject. Natural antimicrobial agents such as spice volatile oils (thyme and garlic), organic acids (acetic, citric, lactic acids) nissan are used to produce antimicrobial packaging materials. Nowadays, nanoparticles using plant extracts have gained attention due to it is a simple and economical method. Previous studies reported that the formation of gold and silver nanoparticles by living plants attained the biosynthesis of metal nanoparticles by plant leaf extracts and explored their potential applications.4 The extract yields and resulting antioxidant activities of the plant materials are strongly based on the nature of extracting solvent, due to the presence of different antioxidant compounds of varied chemical characteristics and polarities that may or may not be soluble in a particular solvent. Polar solvents are frequently employed for the recovery of polyphenols from a plant matrix.5 Gelatin has been attracted the attention for the developments of edible films due to its abundance and biodegradability to improve the water vapour barrier properties of the gelatin films. Gelatin films with strong water vapour barrier properties could be an alternative ecofriendly edible film could be used to preserve the shelf life of foods, especially those sensitive to the quality changes induced by moisture absorption.6 The postharvest life of strawberries can be extending by several techniques combined with refrigeration. Edible films have long been known to protect perishable food products from deterioration. The purpose is to extend the shelf life of produce and to provide a barrier against hazards. It may retard moisture migration and the loss of volatile compounds, reduce the respiration rate, and delay changes in textural properties. Also, it displays an excellent barrier to fats and oils, and has a high selective gas permeability ratio CO2/O2 as compared to conventional synthetic films.7 Strawberries are one of the most popular summer fruits worldwide that are characterized with unique and highly desirable taste and flavour. They are rich in polyphenols and anthocyanins, vitamins and amino acids. The main characteristics related to the quality of the ripe strawberries are their texture, flavour (organic acids and soluble sugars content) and colour.8,9 The objective of this work is extraction silver AgNo3 and extract luria leaves/acetic acid glacial synthetic nanoparticles loaded on gelatin films, and physical & mechanical, viscosity, vapour, zeta particle size emulsion and SEM to select the best edible coating previous check to improve strawberry fruit storability under cold storage. Also, it is aimed to minimizing decay and microbial growth during the storage period.
Materials
Luria leaves were obtained from the Rajab Al-attar shops. The gelatin powder nanoparticles are used to produce gelatin films. Chemicals were experiment, ethyl alcohol 98% and methanol from el nasr Chemicals Company, Hydrochloric Sulphuric acid from el-Gomhouria Company. Glycerol and sodium hydroxide from Acmatic company, Cairo, Egypt). Acetic acid glacial, sorbitol, Whitman no: 1 filter paper and decanter 50 mesh across–organics, Company New Jersey USA. Gelatin powder, win Lab Company, (UK) .Tween 80 from Company China, Silver nitrate AgNo3 from Jenapharm, Germany.
Prepared crude phenolic compounds extracts from luria leaves
The plant material was extracts with solvents ratio (ethanol and methanol: water , 80: 20 v/v ) (200 ml ) for 6 hours in an orbital shaker in a water bath in separate , the residues filtering through Whitman No.1 filter paper.The residues were extracted twice with the same fresh solvent and extracts combined. The solvent under reduced pressure at 45°C using a rotary evaporator (-40°C) according to Bushra et al.,(2009).5 In this part of investigation, we used crude phenolic compound extracts are prepared by adding (0.5 to 4.5%) acetic acid glacial adhesion the described film formation solution mentioned above was modified. Which were acidified with 1-5% acetic acid and maceration was conducted for 1h at ambient temperature in a dark place according to Nyi Mekar Saptarinil, et al. (2018), Olafsson., et al (1993) and Nelson et al., (2016).10,11,12
Characterization of silver nanoparticles
Preparation of Silver nano particles by extract 5 ml extract luria leaves was added into 45 ml 0.002 M AgNO3 solution in 100 ml conical flasks at room temperature in dark for some period. After one hour, formation of silver particles started according to Vankar and Shukla (2012).4
Preparation of Film-Forming Solution
Gelatin powder (8g was dissolved in 100 mL of distilled water (at room temperature) and the mixture was stirred until the gelatin completely dissolved (approximately. 15 min). Add 1.2 grams of glycerol and D-sorbitol were then added to the gelatin film-forming solution, Solution pH was adjusted to pH 7 using sodium hydroxide (2N). Then, the solution was heated to 85°C for 15 min. Then, add extract luria leaves / glacial acetic acid (0.5/4.5) and silver AgNo3 in a ratio of 0.25, 0.50, 0.75 and 1.0 ml to the gelatin solution preparation of edible film nanoparticles. To stabilize the emulsion at crude phenolic compounds extracts (as antioxidants), Tween-20 was also added to the gelatin solution with a ratio of 0.2% of the crude phenolic compounds extracts (as antioxidants). Then the film-forming solution with crude phenolic compounds extracts (as antioxidants) was incorporated and the mixture was homogenized at 6.000 rpm for 2min and sonication for 15min. After cooling to room temperature, the film forming solutions (40ml) were casted on 20×20 cm plates, and then dried for 4 days at ambient conditions (27±10C).6
Ten groups were created as follows
The mentioned solutions were modified by adding 0.25, 0.50, 0.75 and 1.0 ml for (a, b, c and d) respectively and control, e of mixing extract luria leaves with glacial acetic acid (0.5/4.5) synthetic nanoparticles loaded on gelatin films. While, adding 0.25, 0.50, 0.75 and 1.0 ml for (f, g, h and i) respectively control, j of mixing action silver AgNo3 (1%, w/v) with adding 0.25, 0.50, 0.75 and 1.0 ml from extract luria leaves/acetic acid glacial (0.5/4.5) synthetic nanoparticles loaded on gelatin films.
Divided into ten equal parts
All treatments were prepared mixing extract of luria leaves with glacial acetic acid and or mixing silver AgNo3 with extract luria leaves/glacial acetic acid synthetic nanoparticles were loaded on gelatin films, and then ten treatments were divided into two groups as follow:
Storage treatments of studied strawberry fruits
Strawberries (Fragaria x ananassa cv. Festival), were grown in a local farm on loamy soil (Elkalubia Governorate, Egypt) and received the normal agriculture practice during the two successive seasons (2019). Uniform strawberry fruits, in size and free from physical damage or fungal infection, were harvested at ¾ surface colour stages, packed in field box and transported to the lab. Strawberry was stored overnight at 0.5ºC. In the next day, the strawberry fruits were washed with tap water and then immersed for 2 min. in disinfectant solution of calcium hypochlorite (0.25g L - 1). At Central Lab of Agriculture Res. and Food Tech. Res. Institute, Giza, Egypt to study the effect of different postharvest treatments on quality of fruit.
The different coated groups were dipped for one minute in the edible film mixture. Coated strawberry were drained after dipping and packaged in plastic trays with approximately 250g. After that, all boxes were cold stored at 0.5°C and 90-95% RH for 12 days, and kept in carton boxes all samples were kept after packaging. The cooled storage was carried out in the post-harvest research department, Horticulture research institute, Agriculture research center- Giza. During storage period samples of investigated strawberry were periodically with drown for analysis.
Physical and mechanical and rheological properties of prepared nano technology on edible films
Rheological properties: parameters, shear rate and shear stress to choose the best measured solutions on a Brookfield machine labs DV-III Remoter. The viscometer was operated between 10 and 60 rpm .The Sc 4-25 Spindle was selected for the measurement.
Determination of particles Zeta size: Malvern, United Kingdom, Model: Nano size range (nm): 0.6 6000 nm zeta range (mv): (- 200: 200mv) and XR- Diffraction. Model: XPERT –PRO-P Analytical-Netherland.
Scanning Electron Microscopy: inspect S, TM 1999-2007 B wild date, FEL company Auld number D 8571Machine type inspect S.13
Determination of thickness: The thickness film gelatin was measured using a digital micrometer (mitutoyo digimatic indicator corporation, model: pk- 1012: E, Japan micrometer jaws).14
% Solubility in water: The films nano particles at different treatment (a, b, c, d and e) and (f, g, h, i and j). Dry film sample of 0.5g were immersed in beakers containing 50ml of distilled water for 24 hours with periodical gentle shaker incubator. Films were removed from the water and placed back in the desiccator until constant weight according to Munozetal.,(2004).15
Mechanical properties of prepared nanotechnology on edible films: The tensile properties were measured by a texture analyser CT3. The films were cut into strips 3x5cm. These were gripped at each end by a jaw and then the jaws were moved a part at the controlled speed until was automatically recorded to Hernandez, (2004).16
Water vapor permeability (WVP): The water vapour transmission rate [g/(s.m2)] and water vapour permeability through films was determined gravimetrically using the ASTM method E96-95.
Where, is the moisture gain weight per time (g/s), A is the surface area of the film m2, L is the film thickness (mm) and is the difference in relative humidity (ASTM E96-95).
Measurement of gas permeability: Gas testing instrument, model Witt Oxybaby headspace gas analyser (O2/CO2) following the method described by García et al. (2000).
Where, P is the permeability of gas , (m3/m.day.mm Hg), Q is the quantity of gas diffused m3, X is the thickness of film, A area of the film, m2, t is the time, day and ΔP is the pressure difference across the films
Physico-chemical and microbiological properties
Weight loss, total soluble solids (TSS%) and acidity: were determined according to the methods of AOAC (2010).17
Firmness: Texture was determined by a universal testing machine (Cometech, B type, Taiwan) In Food Technology Research Institute, Giza, Egypt provided with software. An aluminium 25 mm diameter cylindrical probe was used in a “Texture Profile Analysis” (TPA) double compression test to penetrate to 50% depth, at 1 mm/s speed test Firmness (N).18
Colour measurement: Internal colour measurements L and values of strawberry fruits were measured by using Minolta Chroma Meter, Model CR - 200. Calibration was done by a white plate before use. Colour changes were quantified for L value which refers to the lightness, and a value which refers to yellow tonality.19
Microbial analysis
Total microbiological count was determined according to Marshall, (1992)20 all the microbiological counts were carried out in duplicates.
Total plate count: The total colonies of bacteria were estimated using plate count agar medium. The plates were incubated at 37ºC for 48 hours.
Moulds and yeasts count: The mould and yeast were determined using the methods for the microbiological examination of foods described by the American Public Health association (A.P.H.A, 1976)21 by using malt extract agar medium the plates were incubated at 25ºC for 5 days.
Statistical Analysis: The results were analysed statistically on the program(MSTAT) using the method LSD at the level of 5%
Rheological properties of prepared nanomaterial of edible films
The results indicated the rheological properties (a, b, c, d and e ) and (f, g, h, i and j) were measured at room temperatures and various shear rates ( 9.30,18.60,27.90, 37.20, 46.50, 55.80 1/s ). Figure 1 shows the relation between shear rate, shear stress on viscosity of different treatments .The results observed that the samples exhibited non-Newtonian pseudo plastic behaviour and fits the power low well to the following equation
→ (1)
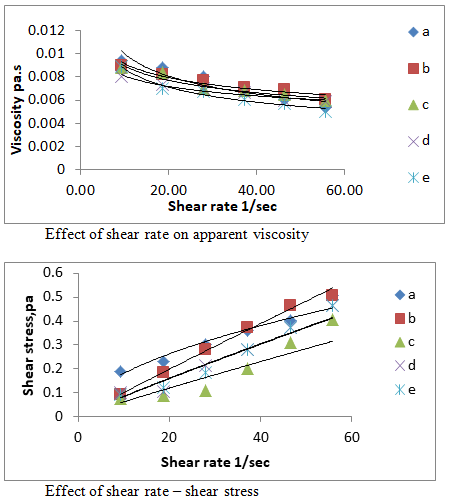
Figure 1 Effect of shear rate on apparent viscosity and shear rate- shear stress at different treatments ( a, b, c, d, e) of edible coating and films nanomaterials.
Where: τ: shear stress, pa; γ: shear rate 1/sec; k: consistency index, n: flow behaviour index. As shear rate increased , the shear stress increases at different treatments (a, b ,c, d, e, f, g, h, i and j) ,while viscosity decreased as shear rate increased as shown in Figure 1 & 2 and Table 1 shows the relation between (k),(n) and treatments of nano materials .These findings it was possible to predict that during coating application more viscous solutions would exhibit higher adherence to surfaces than the less viscous ones, which could result in very distinct coating thickness among foods if a dipping process is used.
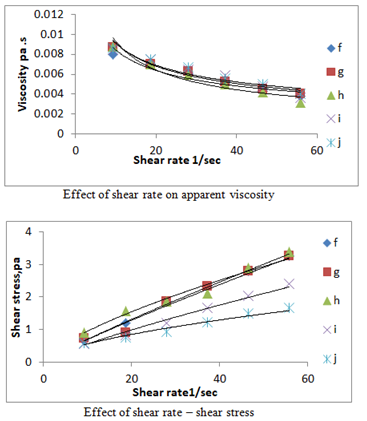
Figure 2 Effect of shear rate on apparent viscosity and shear rate – shear stress at different treatments ( f, g, h, i, j ) of edible coating and films nanomaterials.
|
Formation of antimicrobials action of extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
||||||
|
Treatments |
viscosity |
shear stress |
||||
|
k |
n |
R2 |
k |
n |
R2 |
|
|
a |
0.0204 |
0.309 |
0.8648 |
0.5310 |
0.5339 |
0.9539 |
|
b |
0.0146 |
0.202 |
0.9231 |
0.0109 |
0.9703 |
0.9974 |
|
c |
0.0147 |
0.216 |
0.9395 |
0.0071 |
0.9437 |
0.8323 |
|
d |
0.0121 |
0.173 |
0.9363 |
0.0103 |
0.9190 |
0.9056 |
|
e |
0.017 |
0.290 |
0.970 |
0.0099 |
0.9278 |
0.9456 |
|
Formation of antimicrobials action of silver Ag No3 and extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
||||||
|
f |
0.0214 |
0.404 |
0.9202 |
0.1888 |
0.7019 |
0.9756 |
|
g |
0.0229 |
0.401 |
0.8957 |
0.0887 |
0.8920 |
0.9408 |
|
h |
0.0317 |
0.533 |
0.9133 |
0.1380 |
0.6067 |
0.9659 |
|
i |
0.0245 |
0.428 |
0.8453 |
0.0830 |
0.8267 |
0.9821 |
|
j |
0.0229 |
0.401 |
0.8957 |
0.1380 |
0.6067 |
0.9639 |
Table 1 Relation between consistency index (k) and flow behavior index (n) at different (a, b, c, d and e) and (f, g, h, i and j) of nanomaterial’s of edible films
Enhancement the antibacterial agent of edible coating and films by incorporating it with action of silver Ag No3 with extracts Luria leaves / glacial acetic acid add as a ratio treatments (f 0.25, g 0.50, h 0.75, i 1.0 ml and j control) while mixing extract of luria leaves /glacial acetic acid synthetic nanoparticles treatments (a 0.25, b 0.50, c 0.75 and d 1.0 ml and e control) loaded on nanoparticles gelatin films.
Rheology concerns with the flow and deformation of substances and, in particular, to their behaviour in the transient area between solids and fluids. Moreover, rheology attempts to define a relationship between the stress acting on a given material and the resulting deformation and/or flow that takes place22 Knowledge of the rheological properties of food products is important for design and process evaluation, process control. In addition, the characterization of time-dependent rheological properties of food systems is important to establish relationships between structure and flow(Table 2).23,24
|
Treatments |
Thickness Um |
Tensile strength (N/M2) |
Elongation (%) |
O2 M3.M/M2×10-7day.mmHg |
CO2 M3.M/M2 ×10-8 day.mmHg |
Water vapors [g/m2.24hr] |
% Solubility in water |
||
|
Formation of antimicrobials action of extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||||
|
a |
75 |
176.43 |
75.72 |
28.64 |
26.45 |
11.52 |
24.57 |
||
|
b |
64 |
164.92 |
68.64 |
35.24 |
33.64 |
13.78 |
29.56 |
||
|
c |
56 |
156.24 |
52.75 |
42.4 |
37.22 |
12.98 |
34.1 |
||
|
d |
51 |
142.53 |
50.34 |
48.57 |
40.25 |
14.6 |
36.73 |
||
|
e |
60 |
139.12 |
46.28 |
56.45 |
59.1 |
15.85 |
42.6 |
||
|
Formation of antimicrobials action of silver Ag No3 and extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||||
|
f |
70 |
135.43 |
40.23 |
23.64 |
27.21 |
10.87 |
16.88 |
||
|
g |
62 |
130.21 |
34.45 |
20.54 |
22.67 |
9.23 |
14.65 |
||
|
h |
50 |
127.56 |
31.89 |
18.32 |
14.54 |
8.34 |
13.23 |
||
|
i |
48 |
125.45 |
28.87 |
14.57 |
12.32 |
6.65 |
9.45 |
||
|
j |
59 |
140.34 |
44.45 |
25.45 |
29.45 |
7.67 |
11.43 |
||
Table 2 The thickness, mechanical properties and permeability of nanotechnology on edible films
Enhancement the antibacterial agent of edible coating and films by incorporating it with action of silver AgNo3 with extracts Luria leaves / glacial acetic acid add as a ratio treatments (f 0.25, g 0.50, h 0.75, i 1.0 ml and j control) while mixing extract of luria leaves /glacial acetic acid synthetic nanoparticles treatments (a 0.25, b 0.50, c 0.75 and d 1.0 ml and e control) loaded on nanoparticles gelatin films.
Physical and mechanical properties of different nanotechnology on edible coating & films
These results are in agreement with (2), It was found that the decrease in values was film thickness (i 48, h 50, d 51, c 56 and j 59 um) , tensile strength( i 125.45, h 127.56, g 130.21, f 135.43 and e 139.12 .M.M2) , elongation(i 28.87, h 31.89, g 34.45, f 40.23, j 44.45 %), (Oxygen i14.57, h18.32, g 20.54 , f 23.64, j25.45 M3.M/M2 X10-8) , (CO2 i 12.32, h14.54, g22.67, f 27.21, j29.45M3.M/M2 X10-8) water vapour permeability [ i6.65, j7.67, h8.34, g9.23, f10.87 [g/m2.24hr] and solubility (i 9.45, j 11.43, h 13.23, g 14.65, f 16.88 %). While, the values were high in the treatment was film thickness (e 60, g 62, b 64, f 70 and a 75 um ) tensile strength (a 176.43, b 164.92 N.M.M2), elongation (a 75.72,b 68.64 %), Oxygen (d 48.57, e 56.45 M3.M/M2 X10-7), CO2 (d40.25,e 59.10 M3.M/M2. X10-8), water vapour permeability [d14.60, e15.85 [g/m2.24hr] and solubility (d 36.73,e 42.60 %). Such results are in agreement with those obtained by Danijela Z. Šuput1, et al (2016) and Franciele et al.,(2013). The mechanical properties of the edible coating and film Produced from the chitosan had an 18.5% increased elongation than the chitosan with protein quinoa film and in added to combine the nano particles with oil thyme, this reduce the chitosan film.12
Nanotechnology measurements in solutions in the specified proportions
Particle size: The results recorded in the Table 3 and Figure 3, indicate that the effect of added nanomaterial's treatments (a, b, c, d and e) of edible solution supplement with nano materials on the properties of the definitions of solution evaluated on the change in size and the z-potential of the nanoparticles relative to the films nanomaterials, polydispersity index (pdI) in the peak was 0.429,0440,0.198,0.224 and 0.256 for (a, b, c , d and e) respectively as well as diameter of particle size in the peak 3 was 332.3,407.0,411.0,577.8 and 660.2 for (a, b, c, d and e) respectively. While it was found that the Figure 4 & 5 (PdI) in the peak was 1.000,0.655,0.983,0.981 and 0.604 for (f, g, h, I and j) respectively as well as diameter of particle size in the peak 5was 17.7, 14.50, 12.64, 5.70 and 71.26 for (f, g, h, I and j) respectively. Where he found that the concentration of silver nitrate AgNo3 and extract luria leaves in treatments (f, g, h, I and j) was better than other extract luria leaves / acetic acid glacial (a, b, c, d and e) Which improves the properties of edible coating films by incorporating it with green metallic nanoparticles using laurus nobilis leaves. whereas synthesized silver nanoparticles measured 20–30nm in size while, the control silver nitrate obtained was greater than 1000nm size according to Krishnaraj et al (2010).1 It was observed a decrease of about 200 nm on the bentonite particle size in the aqueous solution, as an effect of the undertaken treatment, showing a minimum size of about 450 nm. The decrease in particle size also reduced the Zeta potential value. It is known that the magnitude of such Zeta potential is an indication of the potential stability of colloidal systems. All particles in suspension with a Zeta potential higher than +30 or lower than -30 mV, tend to repel each other, being considered stable. But if its Zeta potential lies between +30 to -30mV, they tend to attract each other and to flocculate.24,25
Treatments |
particle size distribution(nm) |
Zeta potential(mv) |
||
poly dispersity index (PdI) |
hydrodynamic diameter (nm) |
z- potential |
z- deviation |
|
a |
0.429 |
332.3 |
13.4 |
2.97 |
b |
0.44 |
407 |
14.5 |
3.3 |
c |
0.198 |
411 |
17.7 |
4.06 |
d |
0.224 |
577.8 |
15.7 |
2.68 |
e |
0.256 |
660.2 |
20.2 |
4.02 |
f |
1 |
17.7 |
5.58 |
2.68 |
g |
0.655 |
14.5 |
5.13 |
2.67 |
h |
0.983 |
12.64 |
6.03 |
3.46 |
i |
0.981 |
5.7 |
5.65 |
3.35 |
j |
0.604 |
71.26 |
5.48 |
3.11 |
Table 3 Measured particles size and zeta potential of nanotechnology on edible films formed from it
Enhancement the antibacterial agent of edible coating and films by incorporating it with action of silver Ag No3 with extracts Luria leaves / glacial acetic acid add as a ratio treatments (f 0.25, g 0.50, h 0.75, i 1.0 ml and j control) while mixing extract of luria leaves /glacial acetic acid synthetic nanoparticles treatments (a 0.25, b 0.50, c 0.75 and d 1.0 ml and e control) loaded on nanoparticles gelatin films.
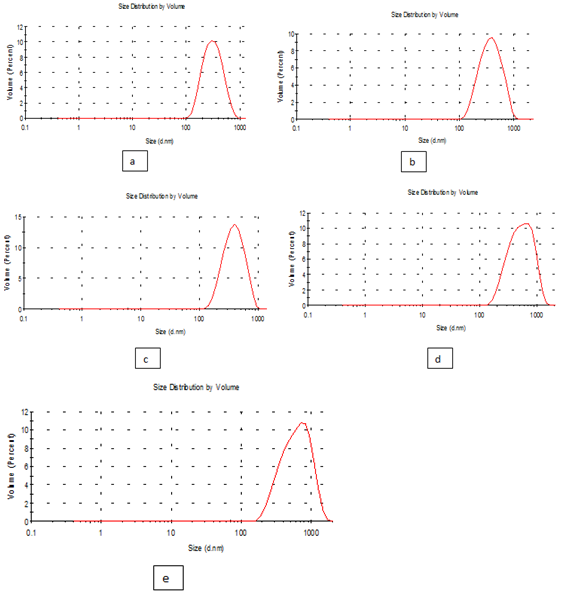
Figure 3 particle size of edible solutions (a, b, c, d and e): formation of antimicrobials action of extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films.
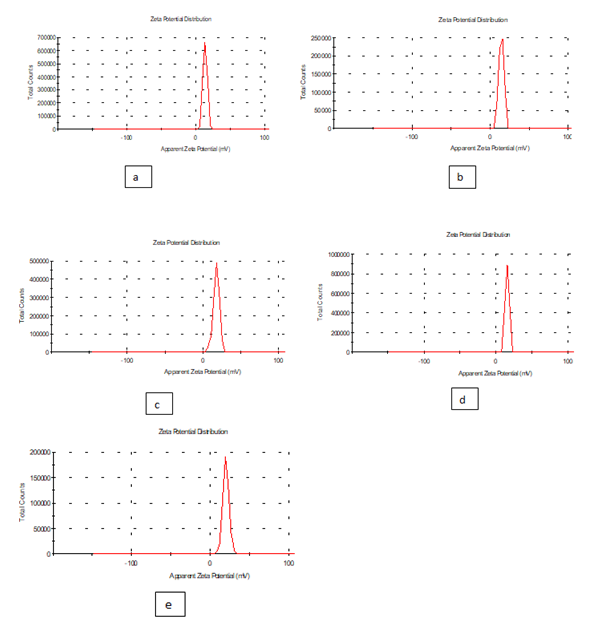
Figure 4 Zeta potential of edible solutions (a, b, c ,d and e): formation of antimicrobials action of extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films.
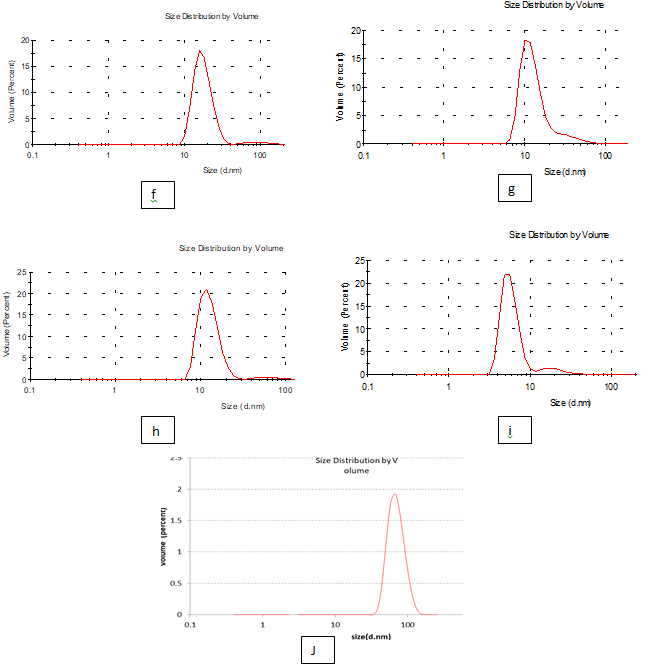
Figure 5 particle size of edible solutions (f, g, h ,i and j): Formation of antimicrobials action of silver Ag No3 and extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films.
Zeta potential: It is clear from the results of the study that zeta potential and zeta deviation was measured to determine the stability of nanoparticle, it depends on the of nanoparticle size of samples (a , b , c , d and e) of edible films nanomaterial’s, solution as shown in Table 3 and Figure 4. The results of zeta potential (mv) and zeta deviation were as follows: (13.4,14.5,17.7,15.7and 20.2) respectively and zeta deviation: (2.97,3.30,4.06,2.68,and 4.02 ) respectively, whereas found that the Figure 6 characterization of the one peak of zeta potential and zeta deviation the area below the curve was 100% found that the contents of zeta potential in transactions on peak ( f , g , h , i and j) had zeta potential (mV) ( 5.58,5.13,6.03,5.65 and 5.48 ) respectively , as well as the contents of zeta deviation in transactions on peak ( 2.68,2.67,3.46,3.35 and 3.11 ) respectively. Zeta potential standard the electric charge at the limit of a colloidal particle, and it is an important index for its surface charge. Zeta potential determines the electrostatic repulsion among them and is accountable for their stability against precipitation and it is usually obtained by measuring and converting from the electerophoretic mobility of the particles. As recommended by Zi Teng et al .,(2013) and American Society for Testing and Materials (ASTM,2003),26 zeta potentials with an absolute value of higher than 30 mv are indicative for “moderate to good “ stability of colloidal systems (ASTM). The higher the zeta potential the better the stability of dispersion. A desirable zeta potential Teng et al.,(2015) choosing highly charged polymers (e.g., protein or chitosan) as an encapsulant . Whereas, when poorly charged materials are employed for encapsulation, a second layer of highly charged polymers may be introduced to improve the dispersion stability (Zi Teng, et al.(2015).
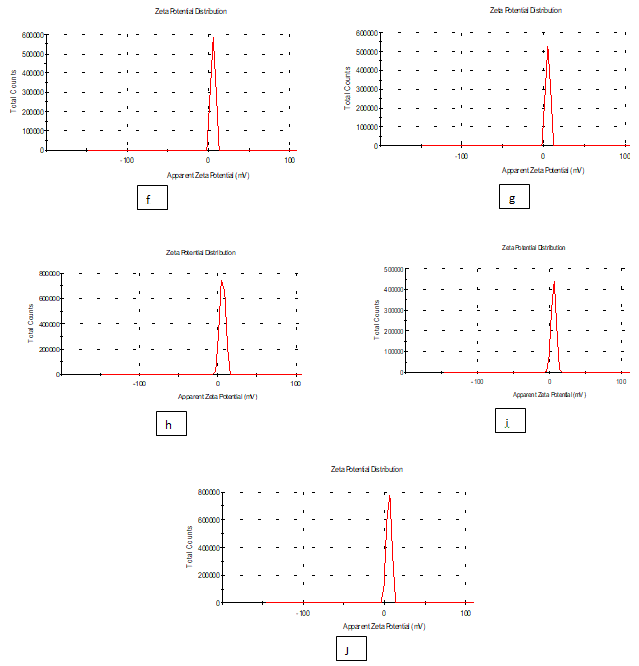
Figure 6 Zeta potential of edible solutions (f, g, h ,i and j): Formation of antimicrobials action of silver Ag No3 and extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films.
Nanotechnology on gelatin films using (SEM) scanning electron microscopy: The microscopic images of ten edible nanoparticle films are presented in figure (a, b, c, d, e, f, g, h, i and j). The edible films give films characterized with flat emerge and clumsy bottom average size from 40 to 50 um polygonal crystals spherical and ellipsoidal morphology. Also contains films has homogeneous structure with some micro granules embedded in a continuous matrix. However, the edible films nano particles produced films the characterized with a smooth surface Figure 7. It was found that by characterizing the image with nano values it was found to increase with (a332.3nm), (b407.0nm), (c411.0nm), (d577.0nm) except for the control much higher (e 660.2nm )) and decreases with (f 17.7nm), (g 14.50 nm), (h 12.64nm), (i5.70nm) except for the control increased (j71.26nm). The preparation of spherical particles of uniform size (200nm) has been found to be rather narrow, with several other kinds of poly disperse aggregates and anisotropic structures being formed above or below the optimum pH 6 the particle size have a wide of shapes and size according to patricia et al (2010).27 Similar to our study, the SEM image the size of the control silver nitrate obtained was greater than 1000nm size, whereas synthesized silver nanoparticles measured 20–30nm in size according to Krishnaraj et al (2010).1
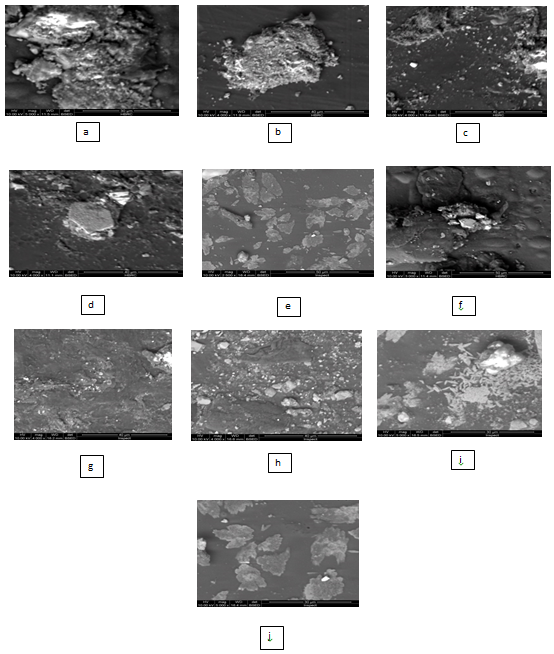
Figure 7 (a, b, c, d, e, f, g, h, i and j) : SEM micrograph of producing Films.
Enhancement the antibacterial agent of edible coating and films by incorporating it with action of silver Ag No3 with extracts Luria leaves / glacial acetic acid add as a ratio treatments (f 0.25, g 0.50, h 0.75, i 1.0 ml and j control) while mixing extract of luria leaves /glacial acetic acid synthetic nanoparticles treatments (a 0.25, b 0.50 , c 0.75 and d 1.0 ml and e control) loaded on nanoparticles gelatin films.
Applications of chosen proper nanomaterial’s of edible films to strawberries fruit
Physico-chemical and microbiological of coated strawberries fruit during storage period
Weight loss percentage: The results indicated in Table 4 found that the treatments (a , b ,c ,d and e) increase the values of weight loss with increasing the storage period at cooled temperatures and also found that the increase in the percentage of loss was in the control . It was also found that the treatments (f, g, h, I and j) were low in the percentage of weight loss compared to other treatments. As noted that the values of the losses are increasing gradually treatments (a, b, c, d, e, f, g, h, I and j) respectively. The basic mechanism of weight loss from fresh fruit and vegetables is by vapour pressure at different locations,28 although respiration also causes a weight reduction.29 This reduction in weight loss was probably due to the effects of the coating as a semi-permeable barrier against O2, CO2, moisture and solute movement, thereby reducing respiration, water loss and oxidation reaction rates.30,31 In both samples kept in packaged plastic trays and with carton boxes and packaged in plastic trays.
|
Treatments |
0 |
2 |
4 |
6 |
8 |
10 |
12 |
|
Formation of antimicrobials action of extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
|
e |
zero |
5.2 |
6.4 |
8.3 |
- |
- |
- |
|
a |
zero |
3.7 |
4.6 |
5.8 |
6.6 |
8.4 |
10.4 |
|
b |
zero |
3.9 |
5.1 |
6.4 |
8.9 |
8.8 |
8.6 |
|
c |
zero |
4.2 |
5.1 |
6.3 |
7.4 |
8.5 |
9.1 |
|
d |
zero |
4.5 |
5.7 |
7.5 |
8.6 |
9.2 |
10.4 |
|
Formation of antimicrobials action of silver Ag No3 and extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
|
j |
zero |
5.1 |
6.3 |
8.2 |
- |
- |
- |
|
f |
zero |
3 |
4.2 |
5.6 |
6.3 |
8.3 |
10.2 |
|
g |
zero |
3.4 |
5 |
6.2 |
7.4 |
8.5 |
9.2 |
|
h |
zero |
3.5 |
4.5 |
5.6 |
6.3 |
7.8 |
9.6 |
|
i |
zero |
3.8 |
4.9 |
6 |
6.4 |
7.6 |
9.1 |
|
L.S.D. |
S = 1. 32 |
T = 1. 93 |
S&T = 0.14 |
||||
Table 4 Effect of edible coatings on weight loss (%) of strawberries fruit during storage
(-) a spoiled reject samples, e (control), j (control)
LSD Treatments = T LSD Storage period = S LSD (Storage period* Treatments) = T * S
Means within a column showing the same letters are not significantly different (P≥ 0.05).
Enhancement the antibacterial agent of edible coating and films by incorporating it with action of silver Ag No3 with extracts Luria leaves / glacial acetic acid add as a ratio treatments (f 0.25, g 0.50, h 0.75, i 1.0 ml and j control) while mixing extract of luria leaves /glacial acetic acid synthetic nanoparticles treatments (a 0.25, b 0.50, c 0.75 and d 1.0 ml and e control) loaded on nanoparticles gelatin films.
Total soluble solids (TSS): In general, there was a gradual increase in total soluble solids (TSS) during the complete storage period at cooled temperatures (Table 5) the TSS was reduction in control as compared to other treatments. As noted the values of the increasing gradually treatments (a, b, c, d, e, f, g, h, I and j ) respectively. Decreased respiration rates also slow down the synthesis and use of metabolites resulting in lower (TSS).28 Coating film on the surface of strawberry reduced respiration rate and vital process, thus reducing the loss of TSS during storage.32 The interaction between treatments and storage period was not significant in both seasons.
Treatments |
0 |
2 |
4 |
6 |
8 |
10 |
12 |
Formation of antimicrobials action of extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
e |
4.5 |
5.6 |
6.4 |
7.8 |
- |
- |
- |
a |
4.6 |
5.7 |
6.6 |
7.9 |
9.2 |
9.4 |
9.9 |
b |
4.7 |
5.8 |
6.5 |
8.1 |
9.1 |
9.3 |
9.5 |
c |
4.8 |
5.9 |
6.7 |
7.9 |
8.2 |
8.8 |
9.1 |
d |
4.9 |
6 |
6.8 |
7.8 |
8.6 |
9.2 |
8.9 |
Formation of antimicrobials action of silver Ag No3 and extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
j |
4.5 |
5.7 |
6.5 |
7.9 |
- |
- |
- |
f |
4.4 |
5.5 |
6.3 |
7.5 |
8.5 |
9.2 |
10.8 |
g |
4.3 |
5.3 |
6.0 |
7.1 |
8.2 |
9.0 |
10.7 |
h |
4.1 |
5.0 |
5.7 |
6.5 |
7.4 |
8.2 |
9.8 |
i |
4.0 |
4.8 |
5.5 |
6.2 |
7.0 |
8.0 |
10.2 |
L.S.D. |
S = 1. 20 |
T = 1. 13 |
S&T = 0.22 |
||||
Table 5 Effect of edible coatings on total soluble solids (TSS) of strawberries fruit during storage
(-) a spoiled reject samples, e (control), j (control) LSD Treatments = T LSD Storage period = S LSD (Storage period* Treatments) = T * S Means within a column showing the same letters are not significantly different (P≥ 0.05).
Enhancement the antibacterial agent of edible coating and films by incorporating it with action of silver Ag No3 with extracts Luria leaves / glacial acetic acid add as a ratio treatments (f 0.25, g 0.50, h 0.75, i 1.0 ml and j control) while mixing extract of luria leaves /glacial acetic acid synthetic nanoparticles treatments (a 0.25, b 0.50, c 0.75 and d 1.0 ml and e control) loaded on nanoparticles gelatin films.
Total acidity: The changes in total acidity of strawberries were determined during storage period at cooled temperatures. The obtained results are recorded in Table 6. The results indicated that the total acidity gradually decreased with increasing of storage period at cooled temperature. It is also considered that coatings reduce the rate of respiration and may therefore delay the utilization of organic acids.28 Retention of acidity has been reported previously for various strawberries fruit treated with edible coatings and films Gomaa et al., (2016).33 Acidity retention was reported by Tanada-Palmu and Grosso, (2005),32 using strawberry fruits coated with gluten film. Slowing down the strawberry respiration rate by means of an edible coating could explain the delay in the use of organic acid in the enzymatic reactions of respiration Debeaufort., et al (1998).34
Treatments |
0 |
2 |
4 |
6 |
8 |
10 |
12 |
Formation of antimicrobials action of extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
e |
0.35 |
0.32 |
0.3 |
0.28 |
- |
- |
- |
a |
0.34 |
0.31 |
0.29 |
0.26 |
0.25 |
0.24 |
0.23 |
b |
0.33 |
0.3 |
0.28 |
0.25 |
0.24 |
0.23 |
0.22 |
c |
0.32 |
0.29 |
0.27 |
0.24 |
0.23 |
0.22 |
0.21 |
d |
0.3 |
0.27 |
0.26 |
0.23 |
0.24 |
0.23 |
0.22 |
Formation of antimicrobials action of silver Ag No3 and extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
j |
0.35 |
0.33 |
0.32 |
0.29 |
- |
- |
- |
f |
0.34 |
0.32 |
0.31 |
0.3 |
0.27 |
0.25 |
0.24 |
g |
0.32 |
0.3 |
0.3 |
0.28 |
0.28 |
0.26 |
0.23 |
h |
0.34 |
0.29 |
0.31 |
0.29 |
0.27 |
0.22 |
0.2 |
i |
0.33 |
0.28 |
0.3 |
0.28 |
0.26 |
0.24 |
0.23 |
L.S.D. |
S = 1. 24 |
T = 1. 62 |
S&T = 0.20 |
||||
Table 6 Effect of edible coatings on total acidity (%) of strawberries fruit during storage
(-) a spoiled reject samples, e (control), j (control), LSD Treatments = T LSD Storage period = S LSD (Storage period* Treatments) = T * S, Means within a column showing the same letters are not significantly different (P≥ 0.05).
Enhancement the antibacterial agent of edible coating and films by incorporating it with action of silver Ag No3 with extracts Luria leaves / glacial acetic acid add as a ratio treatments (f 0.25, g 0.50, h 0.75, i 1.0 ml and j control) while mixing extract of luria leaves /glacial acetic acid synthetic nanoparticles treatments (a 0.25, b 0.50, c 0.75 and d 1.0 ml and e control) loaded on nanoparticles gelatin films.
Colour changes: The colour changes was measured recording lightness (L*value), Chroma (intensity of colour) and hue angle (hº). Lightness of the strawberries was affected by storage time Table 7. The results indicated that the strawberries gradually decreased with increasing of storage period at cooled temperatures. The lightness (L*) gradually decreased during storage in both uncoated and coated strawberries. The highest decrease in lightness was observed in uncoated (control, e) and (control, j) strawberries. Changes in the hue-angle (ho) value of coated fruit with storage time were slight and only became significant at the end of the storage period .Chroma was reduced by around 30% for control and 10% for coated fruit. The coating solution gave rise to significant differences in fruit colour by the end of the storage period, surface colour of fruits.35 It is possible that Arabic gum provided a thick barrier against ethylene production and gas exchange between inner and outer environments and therefore delayed the ripening of the fruit during, storage Gomaa et al., (2016).33 These results were agreements with those obtained by Colla et al (2006),36 they found that strawberry fruits treated with edible coating delayed fruits senescence in which the external and internal colour was lighter than that of uncoated fruits. Thus, the senescence delay, evidenced by the decrease in colour changes, demonstrates the effectiveness of this coating.
Treatments |
0 |
2 |
4 |
6 |
8 |
10 |
12 |
Formation of antimicrobials action of extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
e |
95 |
92 |
85 |
81 |
- |
- |
- |
a |
99 |
90 |
86 |
78 |
65 |
45 |
32 |
b |
97 |
78 |
67 |
55 |
44 |
39 |
30 |
c |
94 |
94 |
76 |
67 |
56 |
45 |
32 |
d |
96 |
93 |
96 |
78 |
56 |
67 |
34 |
Formation of antimicrobials action of silver Ag No3 and extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
j |
98 |
78 |
67 |
56 |
- |
- |
- |
f |
109 |
103 |
99 |
88 |
78 |
56 |
34 |
g |
107 |
101 |
87 |
67 |
56 |
53 |
33 |
h |
105 |
99 |
89 |
78 |
59 |
50 |
30 |
i |
103 |
99 |
86 |
70 |
65 |
50 |
34 |
L.S.D. |
S = 1. 12 |
T = 1. 48 |
S&T = 0.16 |
||||
Table 7 Effect of edible coatings on color [hue angle (h◦)] of strawberries fruit during storage
(-) a spoiled reject samples, e (control), j (control) LSD Treatments = T LSD Storage period = S LSD (Storage period* Treatments) = T * S Means within a column showing the same letters are not significantly different (P≥ 0.05).
Enhancement the antibacterial agent of edible coating and films by incorporating it with action of silver Ag No3 with extracts Luria leaves / glacial acetic acid add as a ratio treatments (f 0.25, g 0.50, h 0.75, i 1.0 ml and j control) while mixing extract of luria leaves /glacial acetic acid synthetic nanoparticles treatments (a 0.25, b 0.50, c 0.75 and d 1.0 ml and e control) loaded on nanoparticles gelatin films.
Firmness: From the results of the study in a Table 8 show the effect of using edible coating with nanomaterials on firmness of strawberry during refrigerated storage. The results in Table 8 indicate that the strawberry fruit decreased with increased period of storage days for both treated and control. At the end of storage, control fruit clearly showed the lowest firmness. These results are in agreement with Tanada-Palmu and Grosso (2005),32 they stated that edible coating showed a good result with respect to the retention of fruit firmness probably because this coating slowed down metabolism and prolonged the storage life. Strawberries treated with CaCl2 carried by soy protein or gluten film significantly delayed the loss of fruit firmness compared to control. The favourable effect of CaCl2 treatment in reduction of firmness loss of strawberries during storage may be due to the stabilization of membrane systems and formation of Ca-pectats, which increase the rigidity of the middle lamella and cell wall to increase resistance for polygalacturonase activity Poovaiah.,(1986).37 The interaction between treatments and storage period was significant in the two seasons. In this concern, strawberry fruits treated by soy plus Ca maintained the fruit firmness for 12 days at 0°C, however untreated control maintained the firmness for 6 days from storage, then firmness decrease was more rapidly until the storage end McGuire., (1992).38 It could be responsible for delaying ripening which resulted in the reduction of firmness loss during storage.27 found that increasing respiration activates increased water loss and most likely decreased potential texture depression.
Treatments |
0 |
2 |
4 |
6 |
8 |
10 |
12 |
Formation of antimicrobials action of extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
e |
39 |
30 |
28 |
24 |
- |
- |
- |
a |
40 |
37 |
35 |
30 |
25 |
22 |
20 |
b |
42 |
42 |
34 |
32 |
30 |
21 |
19 |
c |
41 |
40 |
36 |
34 |
30 |
28 |
25 |
d |
40 |
37 |
34 |
32 |
29 |
27 |
25 |
Formation of antimicrobials action of silver Ag No3 and extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
j |
39 |
32 |
25 |
24 |
- |
- |
- |
f |
40 |
30 |
28 |
21 |
18 |
16 |
14 |
g |
41 |
38 |
36 |
32 |
28 |
26 |
24 |
h |
40 |
35 |
32 |
30 |
24 |
22 |
21 |
i |
39 |
34 |
33 |
31 |
25 |
21 |
20 |
L.S.D. |
S = 1. 20 |
T = 1. 54 |
S&T = 0.14 |
||||
Table 8 Effect of edible coatings on Firmness (N) of strawberries fruit during storage
(-) a spoiled reject samples, e (control) , j (control) LSD Treatments = T LSD Storage period = S LSD (Storage period* Treatments) = T * S Means within a column showing the same letters are not significantly different (P≥ 0.05).
Enhancement the antibacterial agent of edible coating and films by incorporating it with action of silver Ag No3 with extracts Luria leaves / glacial acetic acid add as a ratio treatments (f 0.25, g 0.50, h 0.75, i 1.0 ml and j control) while mixing extract of luria leaves /glacial acetic acid synthetic nanoparticles treatments (a 0.25, b 0.50, c 0.75 and d 1.0 ml and e control) loaded on nanoparticles gelatin films.
Total count: The use of edible coating with nanomaterials on which have antimicrobial activity extend the shelf-life of strawberry. The results are shown in Table 9 shows the changes in total bacterial counts of strawberry coated with nanomaterials during storage periods at 0.5ºC.The data indicates that total counts gradually increased with increasing the cold storage period of strawberry in both (a ,b ,c ,d and e) and (f ,g ,h ,i and j) of edible films nanomaterials. The results in Table 9 showed that the microbial load increases with the increase of storage in all storage treatments and the increase in the control is higher than other treatments. Storage lasted only 6 days compared to treatments 12 days of storage. It was also found that the treatments (f, g, h, I and j) are lower in microbial load than treatments (a, b, c, d and e) . The counts reached to 10.89–12×101 CFU/g after 12 days of storage for in both coated and uncoated samples , as compared with the initial counts 0.35×101 CFU/g.
Treatments |
0 |
2 |
4 |
6 |
8 |
10 |
12 |
Formation of antimicrobials action of extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
e |
0.35 |
3.67 |
5.78 |
7.9 |
- |
- |
- |
a |
0.35 |
2.25 |
4.35 |
6.5 |
8.45 |
9.75 |
11 |
b |
0.35 |
2.34 |
4.46 |
6.67 |
8.56 |
10 |
11.34 |
c |
0.35 |
2.4 |
4.52 |
6.73 |
8.9 |
10.23 |
11.58 |
d |
0.35 |
2.45 |
4.67 |
6.89 |
9.14 |
10.68 |
12 |
Formation of antimicrobials action of silver Ag No3 and extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
j |
0.35 |
3.65 |
5.34 |
7.79 |
- |
- |
- |
f |
0.35 |
2.13 |
4.2 |
6.34 |
8.1 |
9.34 |
10.89 |
g |
0.35 |
2.34 |
4.23 |
6.21 |
8.24 |
9.9 |
10.95 |
h |
0.35 |
2.4 |
4.34 |
6.23 |
8.37 |
10.12 |
11.23 |
i |
0.35 |
2.45 |
4.6 |
6.89 |
8.85 |
10.56 |
11.45 |
L.S.D. |
S = 1. 86 |
T = 1. 48 |
S&T = 0.39 |
||||
Table 9 Effect of edible coatings on total count of strawberries fruit during storage
(-) a spoiled reject samples, e (control) , j (control) LSD Treatments = T LSD Storage period = S LSD (Storage period* Treatments) = T * S Means within a column showing the same letters are not significantly different (P≥ 0.05).
Enhancement the antibacterial agent of edible coating and films by incorporating it with action of silver Ag No3 with extracts Luria leaves / glacial acetic acid add as a ratio treatments (f 0.25, g 0.50, h 0.75, i 1.0 ml and j control) while mixing extract of luria leaves /glacial acetic acid synthetic nanoparticles treatments (a 0.25, b 0.50, c 0.75 and d 1.0 ml and e control) loaded on nanoparticles gelatin films.
The reduction of microorganisms in strawberries treated with antimicrobial activity Shelef (1994),39 which provides a protective antimicrobial barrier against food borne pathogens in product Weaver and Shelef (1993).40 Also, micro flora is usually restricted to fungal and lactic acid bacteria when pH was low.41
Moulds and yeast
The changes in moulds and yeast counts of fresh strawberry were determined during cold storage. The obtained results are shown in Table 10, The results indicated that the moulds and yeast counts gradually increased with increasing the storage period at cold temperature in both samples packaged in coated and uncoated forms. The samples uncoated indicates higher mould and yeast counts than coated ones. The moulds and yeast counts reached to 8.45–9.85×101CFU/g after 12 days of storage for in both coated and uncoated samples, as compared with the initial counts 0.12 × 101 CFU/g. These materials offer the possibility of obtaining thin films and coatings to cover fresh or processed foods to extend their shelf life. Edible films and coatings offer extra advantages such as edibility, biocompatibility, esthetic appearance, barrier to gasses properties, non-toxicity, non-polluting and its low cost. In addition, bio-films and coatings, by themselves are acting as carriers of foods additives (i.e.: antioxidants, antimicrobials), and have been particularly considered in food preservation due to their ability to extend the shelf life.42
Treatments |
0 |
2 |
4 |
6 |
8 |
10 |
12 |
Formation of antimicrobials action of extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
e |
0.12 |
2.85 |
4.85 |
8.56 |
- |
- |
- |
a |
0.12 |
1.33 |
2.15 |
3.32 |
6.68 |
7.23 |
9 |
b |
0.12 |
1.45 |
2.23 |
4.34 |
5.78 |
6.68 |
8.45 |
c |
0.12 |
1.7 |
2.46 |
4.85 |
6.9 |
7.79 |
9.67 |
d |
0.12 |
1.85 |
2.68 |
3.99 |
5.1 |
7.24 |
9.85 |
Formation of antimicrobials action of silver Ag No3 and extract luria leaves / acetic acid glacial synthetic nanoparticles loaded on gelatin films |
|||||||
j |
0.12 |
1.74 |
4.75 |
8.45 |
- |
- |
- |
f |
0.12 |
1.45 |
2.15 |
3.18 |
5.12 |
6.95 |
9.68 |
g |
0.12 |
1.23 |
2.95 |
4.89 |
6.9 |
7.85 |
9 |
h |
0.12 |
1.14 |
2.68 |
5.65 |
6.54 |
7.34 |
8.75 |
i |
0.12 |
1.05 |
2.32 |
4.25 |
5.23 |
6.23 |
8.24 |
L.S.D. |
S = 1. 70 |
T = 1. 34 |
S&T = 0.25 |
||||
Table 10 Effect of edible coatings on moulds and yeast of strawberries fruit during storage
(-) a spoiled reject samples, e (control) , j (control) LSD Treatments = T LSD Storage period = S LSD (Storage period* Treatments) = T * S Means within a column showing the same letters are not significantly different (P≥ 0.05).
Enhancement the antibacterial agent of edible coating and films by incorporating it with action of silver Ag No3 with extracts Luria leaves / glacial acetic acid add as a ratio treatments (f 0.25, g 0.50, h 0.75, i 1.0 ml and j control) while mixing extract of luria leaves /glacial acetic acid synthetic nanoparticles treatments (a 0.25, b 0.50, c 0.75 and d 1.0 ml and e control) loaded on nanoparticles gelatin films.
It could be concluded that gelatin films the best samples treatment were AgNo3 nanoparticles action of extract Luria leaves / glacial acetic acid loaded on gelatin films treatments (f, g, h and i ) 17.7, 14.50, 12.64 and 5.70 respectively followed by samples extract of luria leaves /glacial acetic acid synthetic nanoparticles loaded on gelatin films treatments ( a, b, c and d ) 332.3, 407.0, 411.0 and 577.0 respectively as compared with control (e 660.2 and j 71.26) were chosen to the rheological and mechanical properties , permeability, particle and zeta potential emulsion and scanning electron microscopy. He found that the control (e and j) was spoiled reject samples after 6 days while the remaining of the treatments lasted up to 12 days in strawberries. Samples coated with (f, g, h, i) reduced the weight loss and microbial count. The effect of adding AgNo3 with extract Luria leaves/glacial acetic acid nano materials on gelatin films to prolong extend product shelf life and reduce the risk of microorganism’s growth and improved quality on strawberries.43–47
None.
None.
The authors declare that there was no conflict of interest.

©2020 Bakhy, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.