MOJ
eISSN: 2381-182X


Research Article Volume 7 Issue 1
1School of Food and Biological Engineering, Jiangsu University, China
1School of Food and Biological Engineering Jiangsu University China
2Department of Nutrition & Food Technology, Omdurman Islamic University, Sudan
2Department of Nutrition Food Technology Omdurman Islamic University Sudan
3Department of Food Process Engineering, Addis Ababa Science and Technology University, Ethiopia
3Department of Food Process Engineering Addis Ababa Science and Technology University Ethiopia
4Sugar institute, University of Gezira, Sudan
4Sugar institute University of Gezira Sudan
5Department of Food and Dairy Sciences and Technology, Arish University, Egypt
5Department of Food and Dairy Sciences and Technology Arish University Egypt
Correspondence: Mohammed Abdalbasit A Gasmalla School of Food and Biological Engineering Jiangsu University 301 Xuefu Road Zhenjiang Jiangsu 212013 China
Received: January 22, 2019 | Published: February 6, 2019
Citation: Gasmalla MAA, Admassu H, Musa A, et al. Effects of ultrasound pretreatment with different frequencies on the degree of hydrolysis and ACE inhibitory activity of stevia protein. MOJ Food Process Technol. 2019;7(1):11-14. DOI: 10.15406/mojfpt.2019.07.00212
The effects of ultrasound pretreatment with different frequencies and operational modes, including single frequency ultrasound and dual-frequency ultrasound on the degree of hydrolysis (DH) of stevia protein (SP) and angiotensin-I-converting enzyme (ACE) inhibitory activity of SP hydrolysate were investigated. The results of this study indicated that ultrasound pretreatment did not influence DH of SP significantly However, all the ultrasound pretreatment influence the ACE inhibitory activity of SP hydrolysate significantly. The maximum DH under ultrasonic treatment with ideal settings was found to be 11.6% .The dual frequncy ultrasound pretreatment of 20/28 kHz showed higher ACE inhibitory activity compared to that of other single frequency ultrasound and control. In conclusion, the perfect choice of frequency ultrasound pretreatment of SP is indispensable for the preparation of ACE inhibitory peptide.
Keywords: ultrasound frequency, degree of hydrolysis, stevia protein, inhibitory, peptide
Stevia rebaudiana Bertoni is a branched dense belonging to the Asteraceae family which is origin to the Paraguay. It leafs haves rich amount of steviol glycosides. Only rebaudiana and phlebophylla species produce steviol glycosides among 230 stevia genus. Nine steviol glycosides have been identified from leafs of Stevia: stevioside, steviolbioside, rebaudioside A, B, C, D, E, F and dulcoside.1 Even though leafs of stevia rebaundiana were used centuries in South America, only highly purified steviol glycosides were considered as generally recognized as safe in USA 2008 and 2009.2 Acceptable Daily Intake (ADI) for steviol glycosides as reported by joint expert committee for food additives is 0-4 mg/kg BW/day.3,4 Totally steviol glycosides are perfect to use as food additives in the food industry because of its high stability for heat and pH. They, indeed, are broadly used in food products, such as soft and fruit drinks, dessert, sauces, sweet corn etc.2 Using steviol glycosides continuously is very helpful to decrease the level of sugar content and cholesterol in the blood. Therefore, steviol glycosides are well thought-out beneficial for health.1 At present, it is common to use herbal and drug substitute to treat diabetes, and extract of stevia rebaundiana was used to treat diabetes in USA for long time, hence, steviol has possible in drug carriage. Reb A, steviol and stevioside may also have therapeutic applications, which have influence on glucose level of plasma by modulating of insulin secretion and sensitivity; they also inhibit glucose absorption in intestinal and glucose neogenesis by the liver. However, these affects on glucose level of blood can only be observed when plasma glucose level is elevated.5 Hypertension is a chronic health condition and affects the adult’s people all over the world and even threaten our lives, especially for the elderly. Hypertension can cause stroke, chronic kidney disease, heart failure and many kinds of problems.1,6 It is well known that angiotensin-I-converting enzyme (ACE) can transform angiotensin-I into angiotensin-II, temporarily it can also cut bradykinin into inactive fragments to rise blood pressure. Therefore, angiotensin converting enzyme inhibitor can inhibit the activity of ACE to achieve the purpose of regulatory blood pressure.2 Lately, a lot of researchers tend to study on ACE inhibitor peptides from plant protein, which has various bioactive components and has no side effects.3 Enzymatic hydrolysis was extensively used to produce a variety of bioactive peptides from different food sources such as milk protein concentrates, egg rice protein, soybean protein hydrolysate, and so on.7 By enzymatic hydrolysis, the physical and chemical properties as well as the micro structure of proteins can be changed. The molecular conformation of the proteins significantly influences the release of bioactive peptides from food proteins by enzymatic hydrolysis. On the other hand, some proteins are unaffected by enzymolysis due to their compact quaternary and tertiary structures.8 In recent years, ultrasound technology was far and wide applied in food industry, as an innovative non-thermal physical processing technology, particularly in extraction.9,10 Enzymatic hydrolysis,11,12 emulsification.13‒15 Numerous proteins has exposed the increase of bioactive peptide content when use Ultrasound pretreatment before enzymatic hydrolysis. It is supposed that the pretreatment can unfold protein structure and increased accessibility of enzymes to peptide bonds.16 Acoustic cavitation is well-thought-out as the most important factor effected by various ultrasound factors.17 The ultrasound frequency pretreatment is one of the main factors that influence the production of cavitation.18,19 A number of procedures such as heating, high pressure, ultrasound, and microwave have been used to make the proteins more sensitive to enzymolysis. However, between all of these processes, High-intensity/Low-frequency ultrasound (10~100 kHz) was most widely used to modify the protein structure due to cost effective, non-destructive and quick technology.8,20 High intensity ultrasound treatment produces more easy to get to sites of enzymes by changing the protein confirmation through disturbing the quaternary and tertiary structure of globular proteins by influencing hydrogen bonds and hydrophobic interactions.8 Recently, ultrasound technology was successfully applied to improve the DH, functional and bioactive properties.8,21 The objective of this study was to investigate the effect of single and dual ultrasound frequency on DH and. (ACE) inhibitory activity in pretreatment of SP.22
Materials
Stevia leaves powder was purchased from the Yancheng Xiaguang Co. (Jiangsu, China). Alcalase 2.4 L with an activity of 150,000 U/mL (by Folin-phenol method) was purchased from Novozymes Co. Ltd. (China). Angiotensin-I-converting enzyme (ACE) was extracted from the pig lung according to the reported method;23 Hippuryl-His-Leu (HHL) and 1-anilino-8-naphthalene-sulfonate (ANS) were purchased from Sigma-Aldrich Corp (USA). Other chemicals and solvents used in the experiment were of analytical grade.
Stevia protein extraction
Stevia protein (SP) was extracted according to the method described by Jia et al.23 with minor modifications. Stevia leaves powder (50g) was suspended in 1000 mL of distilled water and the pH was adjusted to 9.0 with 3 mol/L NaOH solution and the mixture was stirred at 30°C. Then it was centrifuged at 4000g for 20 min. SP was obtained by adjusting pH of the supernatant to 4.0 with 3.0 mol/L HCl, and the mixture was centrifuged at 4000g for 20 min. The precipitate was re-suspended in distilled water and the pH was adjusted to 7.0. The suspension was dried using a freeze-drier (ALPHA 1-2, Martin Christ Inc., Germany) and kept in sealed plastic bag at 4°C for further analysis.
Different-frequency ultrasound pretreatment of SP
The experiments of ultrasound pretreatments were conducted under different ultrasound frequency modes which were single-frequency (20 and 28kHz), dual-frequency (20 & 28kHz), and the maximum output power and processing capacity of each one was 80W and 1L. The single factor experiment was conducted under the following conditions: SP solution’s volume of 1L, ultrasonic power density of 80W/L, temperature of 30°C and substrate concentration of 50g/L. The ACE inhibitory rate and the degree of hydrolysis (DH) were set as indices for the optimization of ultrasound frequencies and working modes. The SP with a magnetic stirring apparatus without ultrasound under the same conditions was set as control. All experiments were carried out in triplicate.
Enzymolysis hydrolysis method of SP
After ultrasound pretreatment, SP suspension was preheated at 50°C using water bath for 10min and the pH was adjusted to 9.0. Then 31μl alcalase was added to initiate enzymatic reaction. Meanwhile the pH was maintained at 9.0 for 90min by continuously titrated with 1 mol/L NaOH solution. At the end of enzymolysis, the mixtures were boiled in water bath for 10 min to inactivate alcalase. After cooled to room temperature, the hydrolysate was centrifuged at 12,000g for 15 min, and the supernatant was used for subsequent analysis.
Determination of the degree of hydrolysis (DH)
The pH-stat method was used to determinate DH (%) according to the method of Adler-Nissen22 using the following equation:
Where V(ml) is the base of NaOH consumption, N is the normality of the base, α is the average degree of dissociation of the α-NH2 groups in the protein substrate, M is the mass of hydrolyzed stevia protein (g), htot is the total number of peptide bonds in the protein substrate (9.2 mmol/g protein).
ACE inhibitory rate of SP hydrolysate
The ACE inhibitory rate, which is relevant to the antihypertensive activity, was measured according to the previous method23 with some modifications. The hydrolysate was diluted 20 fold by distilled water. The ACE inhibitory rate was formulated by the following equation:
where Sb is a blank peak area of hippuric acid; Si is the hydrolysate peak area of hippuric acid.
The effects of ultrasound pretreatment with different frequencies and working modes on the DH of SP and ACE inhibitory rate of SP hydrolysate
The process parameters were studied and their levels were shown in Table 1. The degree of hydrolysis (DH) is a valid method to determine or measure percent cleavage of peptide bonds in proteins. In this study DH was determined in the SP samples both pretreated with different ultrasonic frequencies and control using the method that described by Adler-Nissen.22 Figures 1-3 showed the degree of hydrolysis (DH) of SP pretreated by ultrasound with different frequencies and operational modes.
Experimental parameters |
Proposed conditions |
Unit |
Sample quantity |
50 |
g |
Enzyme type |
Alcalase |
- |
Enzyme activity |
150,000 |
U/mL |
Temperature |
30 |
°C |
PH |
9 |
- |
Power density |
80 |
W/L |
Single ultrasound frequency |
20 and 28 |
KHz |
Dual ultrasound frequency |
20/28 |
KHz |
Time |
90 |
min |
Table 1 Optimum conditions for hydrolysis of SP
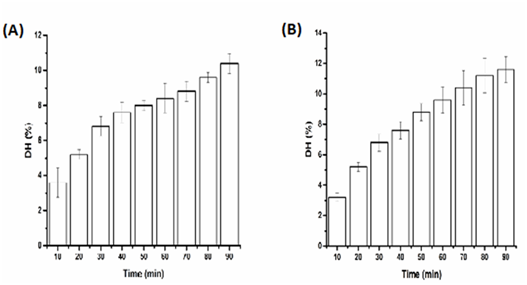
Figure 1 Effects of time on the DH of SP, ultrasound pretreatment with Single frequency (A) 20 KHz and (B) 28 KHz, enzymatic reaction at 50°C and pH9.
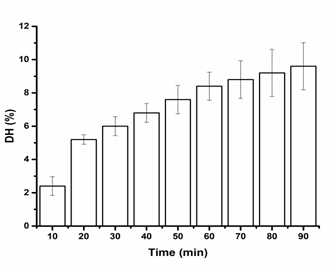
Figure 2 Effects of time on the DH of SP, ultrasound pretreatment with dual frequency 20/28 KHz, enzymatic reaction at 50°C and pH9.
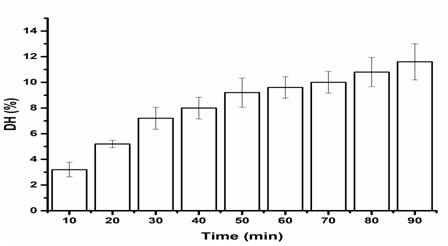
Figure 3 Effects of time on the DH of SP, untreated ultrasound frequency (Control), enzymatic reaction at 50°C and pH9.
Results indicated that all the ultrasound pretreatment did not progress the DH rate which are fall in range 9 to 12 %. The effects of dual frequency ultrasound of two combinations frequency containing 20/28 kHz on the DH of SP resulted value 9.6 % as presented in Figure 2. The degree of hydrolysis of SP went up when the enzymolysis just in progress. As the enzymatic reaction time went on, the degree of hydrolysis has a tendency to be consistent. This may be because that ultrasound pretreatment can destroy partial tight protein structure and makes the protein buried inside primarily expose. This outcome in further effective substrate for the enzyme to interaction the cleavage site when the enzymatic hydrolysis began. Anyway, the DH of SP tends to be consistent with the decrease in the available cleavage site afterwards. And this result is consistent with the result of many literatures.24,25
Figure 4 showed the effects of the single frequency ultrasound pretreatment 20 KHz and 28 KHz, as well as showed dual frequency ultrasound pretreatment 20/28 KHz operating at on the ACE inhibitory activity of SP hydrolysate. The ACE inhibitory rate of SP pretreated by ultrasound with dual frequency ultrasound 20/28 KHz had no significant difference compared with that of 20 kHz (Figure 4) the results That of dual frequency ultrasound with frequency combinations of 20/28 kHz presented higher than other frequency compared with control and two others single frequencies 20 KHz and 28 KHz. It indicated that the ACE inhibitory activity improved significantly by the dual frequency ultrasound compared with the single frequency and control sample. It can be concluded from the results that all the ultrasound pretreatment effected the ACE inhibitory activity of SP hydrolysate. All the ultrasound pretreatment effect on ACE inhibitory activity of SP hydrolysate significantly. The results showed that the ACE inhibitory activity of SP pretreated by 20,28 and 20/28 kHz ultrasound frequency increased by 34.36%, 4.11% and 36.14% respectively, compared with control. The highest ACE inhibitory activity was obtained under 20/28 kHz ultrasound frequency. This result indicated that compared with other ultrasound frequency, 20/28 kHz ultrasound frequency might have generated the change in protein structure which was more suitable to the subsequent enzymatic hydrolysis for preparation of ACE inhibitory peptide.
In this study, all the ultrasound pretreatment with different frequencies and operational modes to be able develop the ACE inhibitory activity of hydrolysate from SP. The 20/28 kHz dual frequency ultrasound presented the highest ACE inhibitory activity of SP hydrolysate. It can be concluded from the outcomes of this study that all the ultrasound pretreatment including single frequency and dual frequency had a significantly influence on the pretreatment of SP.
This research was supported by the post-doctoral station of Jiangsu University, Jiangsu Province, China.
The authors declare no conflicts of interest.

©2019 Gasmalla, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.