MOJ
eISSN: 2381-182X


Review Article Volume 9 Issue 2
Department of Nutritional Science, Faculty of Human Ecology, Yasuda Women’s University, Japan
Correspondence: Akira Nose, Department of Nutritional Science, Faculty of Human Ecology,Yasuda Women’s University,6-13-1,Yasuhigashi, Hiroshima Asaminami-ku, Hiroshima 731-0153, Japan, Tel +81-80-3701-6681
Received: February 23, 2021 | Published: March 10, 2021
Citation: Nose A. Components in water reduce the alcohol-stimulative taste of spirits. MOJ Food Process Technols. 2021;9(2):39-42. DOI: 10.15406/mojfpt.2021.09.00258
Effects of solutes on the stimulative taste of ethanol in transparent spirits such as vodkas were reviewed. It was demonstrated that MgCl2, MgSO4, and NaHCO3 could reduce the alcohol-stimulative taste of spirits and, at the same time, strengthen the hydrogen-bonding structure of water-ethanol in spirits. It was suggested the reduction of the alcohol-stimulative taste is related with a change of the hydrogen-bonding structure in spirits by proton nuclear magnetic resonance (1H NMR) spectroscopy. These salts in spirits seem to be originated in water added to spirits before bottling. Some salts in water could affect the stimulative taste of ethanol in spirits.
Keywords: vodka, Shochu, alcoholic beverage, ethanol, alcohol, water, NMR
We can enjoy tasting many kinds of alcoholic beverages all over the world. They are made by traditional and unique techniques from various ingredients. They have their own characteristics such as flavor, taste, color and so on. Wine and beer are brewages, on the other hand, whisky and vodka are spirits distilled after fermentation. Those spirits do not contain involatile components such as saccharides, amino acids, organic acids. As for whiskies, Scotch, Irish, Bourbon, Canadian, Japanese, distilled spirits are matured for a long time and gained various components from wood casks.1 On the other hand, transparent spirits, such as white rum or vodka are not aged in wood casks. Their tastes are very clear and pure, and these transparent spirits are sometimes called “white spirits”. Many kinds of these white spirits are produced in various regions and have their own characteristics, for example, vodka2,3 (Russia, Eastern Europe), rum4 (Central America), gin5 (Europe), tequila6 (Mexico), Aquavit7 (Northern Europe), Pinga8 (Brazil), Korn9 (Germany), Baijiu10,11 (China). In the manufacturing process of these spirits, a large amount of water is added to spirits to dilute the alcohol content before bottling. Water is a substantial ingredient for those white spirits and the quality of water was considered to be very important for the quality of the spirits products.
The taste of whiskies is very irritative before maturation, but the alcohol-stimulative taste becomes mellow and smooth through aging in wood casks. The change of the alcohol-stimulative taste has been regarded as the result of change in the hydrogen-bonding structure of water-ethanol through hydrogen bonds.12,13 It was reported that the size of clusters through hydrogen bonds related the reduction of the alcohol-stimulative taste of whisky.14 However, the effect of structural change on the change of the taste has not been demonstrated physiologically. We investigated what components contained in alcoholic beverages could change the water-ethanol hydrogen-bonding structure by proton nuclear magnetic resonance (1H NMR) spectroscopy.15 It was found that some ion such as Li+, Mg2+, Ca2+, SO42-, and F- could strengthen the hydrogen-bonding structure of water-ethanol. It was also found that acids could strengthen the hydrogen-bonding structure of water-ethanol, and at the same time, promote the proton-exchange reaction between water and ethanol molecules in ethanol-water solutions. Phenolic compounds such as vanillic acid or gallic acid also showed much stronger effects to strengthen the water-ethanol structure. The degree of the effects of strengthening the water-ethanol structure was related to the acids strength.15 It was also demonstrated that the components strengthened the water-ethanol hydrogen-bonding structure in real products such as whisky,16,17 Japanese sake: a brewage from rice,16,18 and Shochu: a Japanese distilled spirit from various grains16,19 compared to a mere ethanol-water mixture.
Vodka is a very popular spirit all over the world. Vodka products seem to contain much less amounts of chemical components compared to other alcoholic beverages and the taste and the flavor seem to be similar to a neutral alcohol. It seems that the difference of the taste or the flavor between vodka brands is very small. We paid attention to the stimulative taste of ethanol in white spirits such as vodkas and explored components could affect the alcohol-stimulative taste and the hydrogen-bonding structure of water-ethanol. The relation between components and the alocohol-stimulative taste in white spirits was reviewed based on our reports to search for a clue to improve the quality of white spirits.
Sensory evaluation and components of vodkas
The sensory test and analyses for main components were carried out with vodka products purchased in Japan. With the sensory test of the vodkas, the strengths of “alcoholic stimulus,” “sweetness,” “sourness,” “saltiness,” “umami,” “bitterness,” and “astringency” were evaluated compared to a mere ethanol-water solution.20 It was found that the “alcoholic stimulus” was less in vodka products compared to the ethanol-water solution. There was a tendency that “sweetness” of the taste was stronger in those vodkas of which “alcoholic stimulus” was weaker. It seemed that the reduction of “alcoholic stimulus” related to the increase of “sweetness” in vodka products. There was also a tendency that the vodkas showing stronger sweet taste showed stronger “saltiness” of the taste. Main components such as ions, organic acids, saccharides, and flavor components were analyzed with these vodka samples. The vodka samples contained only small amounts of metal cations and anions, the other components such as saccharides or acids could not be detected with all vodka samples although the vodka samples showed sweet taste. These cations and anions seemed to be brought in vodka products from water added to those before bottling. The effects of these ions on the stimulative taste of ethanol were evaluated by checking the taste of the ethanol-water solutions containing a salt.20 This sensory evaluation showed that MgCl2, MgSO4 and NaHCO3 could reduce the stimulative taste of ethanol and that MgCl2 also add the sweetness to the ethanol-water solution. However, MgCl2 itself did not show the sweetness in water. The effect of NaHCO3 reducing the alcohol-stimulative taste was verified in another study.21 The results of the sensory tests with several salts (NaCl, NaHCO3, Na2SO4, KCl, MgCl2, MgSO4, CaCl2) showed that Mg2+, HCO3-, SO42- had the effect of reducing the stimulative taste of ethanol.
Effects of salts on the water-ethanol hydrogen-bonding structure
The effects of the salts reducing the alcohol-stimulative taste on the 1H NMR spectra were compared to the effects of other salts not reducing the stimulative taste. Figure 1 shows the OH proton chemical shifts changes in the 1H NMR spectra of ethanol-water solutions containing a salt. The chemical shift changes toward the lower field (higher chemical shifts values) mean that the hydrogen-bonding structure is strengthened by a salt, and vice versa.22 The chemical shift changes toward the lower field with the increasing concentrations were observed clearly for the ethanol-water solutions containing MgCl2 or MgSO4. NaHCO3 (Figure 1A). These three salts could strengthen the water-ethanol hydrogen-bonding structure in ethanol-water solutions. The other salts seemed to weaken the water-ethanol hydrogen-structure compared to the 15% (v/v) ethanol-water solution. The effects of these salts were also investigated in 40 % (v/v) ethanol-water solutions (Figure 1B). Two peaks of H2O and EtOH could be observed in 40% (v/v) ethanol-water solution (although not in 15% (v/v) ethanol-water solution) and the chemical shift values of the water peaks were only displayed in Figure 1B. The arrows on the figure show the concentrations of salts at which the two peaks of water and ethanol coalesced to a single peak; the proton exchange between water and ethanol molecules was much promoted. With the ethanol-water solutions containing NaHCO3, the two peaks of water and ethanol merged to a single peak at 1×10-3 M and kept the lower-field chemical shift values at 1×10-2 M or 1×10-1M. The two peaks of water and ethanol in the solutions containing MgCl2 or MgSO4 merged at 1×10-1M and showed the chemical shift change toward the lower field similarly to NaHCO3. These distinct chemical shift changes toward the lower field compared to 40% (v/v) ethanol-water solution at 1×10-1M could not be observed for the other salts.
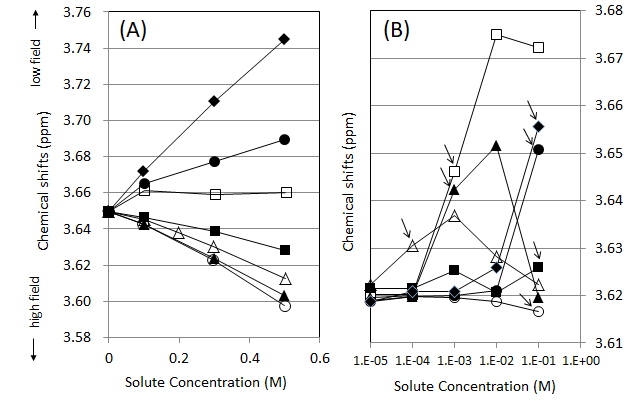
Figure 1 OH proton chemical shifts in 15% (v/v) EtOH-H2O mixtures containing salts (A) and in 40% (v/v) EtOH-H2O mixtures containing salts for lower concentrations below 1×10-1M (B) at 25℃ : (○) NaCl; (□) NaHCO3; (△) Na2SO4; (●) MgCl2; (■) CaCl2; (▲) KCl (◆) MgSO4. The arrows show each point of the two peaks of water and ethanol merging into a single peak.
White column: Both peaks of EtOH and H2O existed separately at 0.5M (0.25M for NaHCO3) in the 1H NMR spectrum. Black column: The peaks of EtOH and H2O merged into a single peak at 0.5M (0.25M for NaHCO3). Coccia et al.22 reported that the strength of the hydrogen-bonding structure varies with the ethanol content in ethanol-water mixtures, and the structure is most strengthened at 20-25% (v/v) in all percentages of ethanol content. The effects of the salts were verified in 25% (v/v) ethanol-water solutions (Figure 2). Figure 2A shows the OH proton NMR chemical shift values in 25% (v/v) ethanol-water mixtures containing each salt. With 600MHz NMR spectroscopy, both OH signals of H2O and EtOH could be observed separately in 25% (v/v) ethanol-water solution at 25℃. The columns were shown in white on Figure 2 when the both peaks of H2O and EtOH existed separately in the 1H NMR spectra, on the other hand, the column in black when the two peaks of H2O and EtOH merged into a single peak. MgCl2, MgSO4, and NaHCO3 showed the larger chemical shift values than 25% (v/v) ethanol-water solution (Figure 2A), that is to say, these salts could strengthen the hydrogen-bonding structure in the ethanol-water solution of the most strengthened structure through hydrogen bonds. All salts investigated here except CaCl2 promoted the interaction between water and ethanol molecules at 0.5M (Figure 2A). To check the effects on the interactions, the similar measurement were carried out at 5℃ at which the proton-exchange reaction between water and ethanol molecules was reduced compared at 25℃ because of the low temperature. MgCl2, MgSO4, and NaHCO3 also showed the larger chemical shift values than 25% (v/v) ethanol-water solution at 5 ℃ (Figure 2B).
However, MgCl2 and MgSO4 did not promote the proton exchange between water and ethanol molecules, NaHCO3 only promoted the proton exchange reaction. We investigated the effects of various salts in 20% (v/v) EtOH-H2O solution, and reported that the intensity of strengthening the hydrogen-bonding structure increased in inverse proportion to the crystal ionic radii per electric charge for cations (i.e., Li+, Na+, Mg2+, and Ca2+) or for anions (i.e., F-, I-, Cl-, Br-, NO3-, ClO4-, and SO42-).15 The results of Figures 1 & 2 shows that MgCl2 and MgSO4 could strengthen the hydrogen-bonding structure of water-ethanol, and that NaHCO3 could promote the interaction between water and ethanol molecules holding the structure strengthened. And at the same time, these three salts reduced the stimulative taste of ethanol organoleptically. This consistency among the results could not be found for any other salts.
Hydrogen-bonding structure of water-ethanol in spirits
1H NMR spectra of two vodka products, 40% (v/v) ethanol-water solution, two Shochu products, and 25% (v/v) ethanol-water solution were shown in Figure 3. The alcohol contents of the vodka products were 40% (v/v) and those of the Shochu products were 25 % (v/v). The spectrum of vodka A showed the two OH peaks of H2O and EtOH distinctly as those of the 40% (v/v) ethanol-water solution, and the chemical shift values of H2O and EtOH peaks for vodka A were just the same as the values for the 40% (v/v) ethanol-water solution, respectively. The hydrogen-bonding structure of water-ethanol of vodka A seemed to be almost similar as that of a mere ethanol-water solution. On the other hand, the peak of ethanol for vodka B seemed to be broader compared to that for the 40 % (v/v) ethanol-water solution. It was also found that the peak of ethanol shifted toward the right side and the peak of water shifted toward the left side, showing that the two peaks nearly coalesced. These changes on the spectrum imply that the interaction between water and ethanol molecules is promoted by the structural change of water-ethanol.19 The hydrogen-bonding structure of water-ethanol in vodka B seemed to be more strengthened compared to the structure in vodka A or 40% (v/v) ethanol-water solution. Three out of nine vodka products showed the similar structure to vodka B, the others were similar to vodka A.20
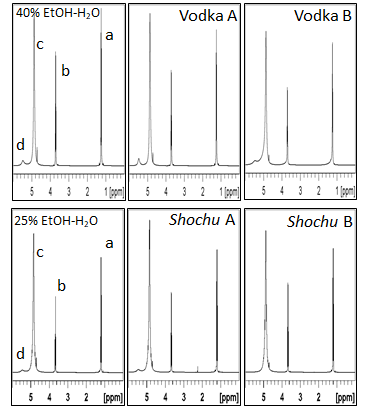
Figure 3 1H NMR spectra of vodka samples, Shochu samples, 40% (v/v) ethanol-water mixture, and 25% (v/v) ethanol-water mixture measured at 25℃. The peaks on the spectrum of 40% (v/v) or 25% (v/v) EtOH-H2O were assigned to CH3 of EtOH (peak a); CH2 of EtOH (peak b); OH of H2O (peak c); OH of EtOH (peak d).
With Shochu products, two peaks of H2O and EtOH were observed separately on the 1H NMR spectrum for Shochu A similarly as were on the spectrum for the 25% (v/v) ethanol-water solution. On the other hand, the two peaks coalesced to a single peak for Shochu B, and the coalesced peak was located toward the left side compared to the water OH peak for the 25% (v/v) ethanol-water solution. It seemed that the water-ethanol hydrogen-bonding structure was more strengthened and the interaction between water and ethanol molecules was more promoted in Shochu B compared to Shochu A or 25% (v/v) ethanol-water solution.
To elucidate the effects of small amounts of components on the alcohol-stimulative taste in spirits, the effects should be clarified quantitatively with analytical methods such as an NMR spectroscopy, at the same time, demonstrated organoleptically by sensory tests. The contents of some cations or anions contained in vodkas were reported.23–25 For other spirits made in various countries, the contents of metal ions were also reported.26–28 These metal ions seemed to originate from water added to the spirits, so the varieties and the contents of these ions were the characteristics of spirits and showed the regional features at the same time.29,30 Various analytical methods were applied for the quantitative determinations of minor components of spirits; gas chromatography-mass spectrometry (GC-MS),31 inductively coupled plasma mass spectrometry (ICP-MS),32 1H NMR spectroscopy,33 1H NMR spectroscopy and sensory test,34 flow injection analysis-isotope ratio mass spectrometry (FIR-IRMS),35 electronic nose coupled to GC,36 electronic tongue.37 Furthermore, some studies have been reported for the water-ethanol hydrogen-bonding structure in spirits. Hu et al. reported the differences in the water-ethanol structure among some vodka products and suggested a relation between the structure and the taste.38 Kuzmin et al. reported the thermodynamic equilibrium of ethanol and water in spirits by means of 1H NMR spectroscopy.39,40 Some components affecting the water-ethanol structure in spirits were reported.41 The solvation of metal ions was reported with aged Shochu in earthenware vessel.42
It was concluded that some salts such as MgCl2, MgSO4, or NaHCO3, in minuscule quantities, could reduce the stimulative taste of ethanol in spirits, at the same time, strengthen the water-ethanol hydrogen-bonding structure. As a result, it was suggested that the reduction of the alcohol-stimulative taste is related with the intimate interaction between water and ethanol molecules through the hydrogen bonding. In the manufacturing process of spirits, these salts seemed to be brought in spirits through diluting water. Needless to say, too much drinking or habitual drinking are undoubtably harmful to our health. White spirits are not mere alcoholic solutions, so they should have more luxurious taste. Water can be an important and valuable ingredient for excellent spirits.
I am grateful to the researchers co-worked for our previous reports, especially Hiroshi Shoji, Yoshihiro Hamakawa, Akira Asahi, Touko Murata, Daisuke Kozaki and Masashi Hojo.
None.
None.

©2021 Nose. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.