MOJ
eISSN: 2381-182X


Research Article Volume 7 Issue 2
1Department of Food Science, Yuanpei University of Medical Technology, Taiwan
2Department of Biotechnology and Pharmaceutical Technology, Yuanpei University of Medical Technology, Taiwan
Correspondence: Chih-Cheng Lin, Department of Biotechnology and Pharmaceutical Technology, Yuanpei University of Medical Technology, No. 306, Yuanpei street, Hsinchu, Taiwan, Tel 886-3-5381183
Received: February 27, 2019 | Published: March 29, 2019
Citation: Lin CS, Hur HF, Lin CC. Antioxidant properties and antibacterial activity of fermented Monascus purpureus extracts. MOJ Food Process Technol.2019;7(2):49-54. DOI: 10.15406/mojfpt.2019.07.00219
Monascus rice ferment extracts has anti-oxidant and antibiotic efficiency. Oxidative stress contributes to skin aging and can adversely affect skin health, which means antioxidants active in skin cells may support skin health. Antioxidant can contribute to defending old. Suppress the production that the epidermis bacterium can reduce the whelk and comedo. Our objective to evaluate the antioxidant properties, antibacterial activity and protective effects on UV-induced damage in HaCaT keratinocytes of extra different carbon and nitrogen sources at water (MRW) and methanolic (MRM) extracts from Monascus purpureus BCRC 31615 fermented rice. Effectiveness in reducing powers and DPPH radical scavenging ability was Monascus rice add MSG water extract has prominent ability. The ferment rice added nitrogen source to increase total phenols and kojic acid content. Antibacterial activities showed good in inhibition Staphylococcus aureus BCRC 10780. Protective effects on UV-induced damage in HaCaT keratinocytes which M. purpureus ferment rice added with extra nitrogen source was especially potent in inhibited UV-induced keratinocyte death.
Keywords: antioxidant, Monascus purpureus, antibacterial, anti-UV, HaCaT keratinocytes
Monascal rice is described as the fermented product of rice on which red mold (Monascus sp.) has been grown. This product has been used in food, as a preservative or to maintain taste and color in fish and meat or for its medicinal properties.1 Reactive oxygen species (ROS) are widely recognized as being involved in the pathogenesis of various diseases and the aging process.2,3 Skin is a major candidate and target of oxidative stress. It is most susceptible to oxidative damage due to the high occurrence of suitable and potential biological targets for such reactions.4 The appearance of human skin is potentially influenced by the balance or equilibrium between two important actions: the rate of growth versus the rate of degradation5,6 The melanocyte is under continuous low-grade oxidative insult. Indeed, melanin synthesis results in the generation of hydrogen peroxide that, if inappropriately processed, can lead to the generation of hydroxyl radicals and other ROS.7 Avoiding ultraviolet (UV) exposure can be inhibited melanin biosynthesis, melancyte metabolism and proliferation.8 Ultraviolet radiation-induce cytotoxic effect mutations have been associated with phenotype and increased risk for skin cancer.9
The antioxidant ability of Monascus metabolites (dimerumic acid, tannin, phenol, etc.) as well as dioscorea was able to perform more antiatherosclerotic effects on increasing total antioxidant status.10 M. anka extract showed antioxidant and hepatoprotective actions. Dimerumic acid isolated from M. anka, that inhibited NADPH and iron (II)-dependent lipid peroxidation (LOP) of rat liver microsomes.11 Monascidin in Monascus can be inhibited the growth of certain bacteria, Bacillus, Streptococcus and Propionibacterium species.12 Staphylococcus aureus and Propionibacterium acnes all cause the epidermis disease.13 If can control these bacteria, can have protective action on the skin health. Cause the skin disease bacterial including Staphylococcus epidermidis, Staphylococcus aureus and Propionibacterium acnes. Monasucs pigment significant antibiotic activities against Bacillus subtilis and Candida pseudotropicalis.14 So it is very important to control these bacteria.
In the present study, the antioxidant and anti bacterial action of Monascus rice extracts was screened, and investigated the protective effects of Monascus rice extracts on UV-induced keratinocyte HaCa T cell line damage. Originally targeted at Staphylococcus aureus, it causes skin irritation, while for Propionibacterium acnes it will cause long acne in the future. Therefore, the two strains were specially selected for determination of minimum bactericidal concentration to confirm the protective effect of the fermentation of Monascus purpures extracts on the skin.
Monascus fermentation was determined according to the method of15,16 Monascus purpureus Went (BCRC 31615) was obtained from the Bioresource Collection and Research Center, Food Industry Research and Development Institute, Hsinchu City, Taiwan. The fungus was inoculated onto Potato dextrose agar (PDA) and incubated at 37°C for 7 days. After pure culture was obtained, the mycelium was re-inoculated into potato dextrose broth and incubated at 37°C for 7 days. The culture was then homogenized in a Waring blender and inoculated into autoclaved rice, at an inoculation rate of 5%. Monascus mycelia was developed on rice mixture supplemented without and with extra 2% carbon source: glucose, glycerol, trehalose, maltose and citric acid; or extra 2% nitrogen source: KNO3 and monosodium glutamate (MSG), respectively, were then produced after the colonization of fungal mycelia for 5, 10 and 15 days at 37°C, respectively. Monascus colonized products that were air-dried in an oven at 40°C. After a fine powder was obtained using a mill, a subsample (40g) was extracted by stirring with 400ml of methanol or water at 25°C at 100 rpm for 24h and filtering through Whatman No. 1 filter paper. The residue was then extracted with two additional 100ml portions of methanol or water as described above. The combined methanol extracts were then rotary evaporated at 40°C to dryness; Water extracts were then freeze dryness. The dried extract was used directly for analyses or stored at -20°C for further uses.
Scavenging ability on 1, 1-dipheny-2-picrylhydrazyl radicals
Each extract in methanol 30μl was mixed with 120μl of water and methanol solution containing DPPH radicals, resulting in a final concentration of 0.2mM DPPH. The mixture was shaken vigorously and left to stand for 30 min in the dark, and the absorbance was then measured at 517 nm against a blank.17 The scavenging ability was calculated as follows:
Reducing power
The reducing power was determined according to the method of Oyaizu.18 Monascus extract in 1 ml of distilled water was mixed with phosphate buffer (1ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (1ml, 1%). The mixture was incubated at 50°C for 20min. A portion (1ml) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10min. The upper layer of the solution (1ml) was mixed with distilled water (1ml) and FeCl3 (200μl, 1%), and the absorbance was measured at 700nm. Increased absorbance of the reaction mixture indicated increased reducing power.
Tyrosinase-inhibition activity was determined by the modified dopachrome method19 using 140μl potassium phosphate buffer (pH 6.8), 100μl sample, and 20μl tyrosinase solution (400 units/ml) were added to a 96-well microplate, which was held for 10 min. The absorbance was measured at 450nm. 20μl L-tyrosine (2.5 mM) added to the 96-well microplate, allowed stand for 20min. The absorbance was measured at 450nm again. The percentage of inhibition of tyrosinase activity was calculated as follows:
A, absorbance of blank solution after incubation; B, absorbance of blank solution before incubation; C, absorbance of sample solution after incubation; D, absorbance of sample solution before incubation.
Determination of total phenolics
The amount of total phenolics in the Monascus extract was determined spectrophotometrically using the previously reported, but modified, Folin–Ciocalteu colorimetric method.20 Briefly, 100μl of the optimal diluted sample was introduced into the test tube. 0.5ml Folin–Ciocalteu phenol reagent was then added to the sample, which was held for 3min. Then, 0.4ml of 7.5% (W/V) aqueous sodium carbonate was added and allowed to stand at room temperature for 30min. The absorbance of the developed color was measured using a spectrophotometer at 765nm. The total phenolics content in each Monascus extract was then calculated by a standard curve prepared with gallic acid and expressed in terms of milligrams of gallic acid equivalents per gram of solid extract.
Determination of kojic acid
The amount of kojic acid was determined according to the method of Kadi 21 prepared kojic acid standard curve (0.1~1mg/ml), expressed in terms of milligrams of kojic acid equivalents per gram of solid extract. Firstly preparation develop discolor reagent (1g FeCl3‧6H2O dissolution in 100ml 0.1N HCl), 25μl kojic acid solution or sample, 100μl FeCl3 solution, and water 125μl were added to a 96-well microplate. The absorbance of the developed color was measured using a spectrophotometer at 500nm. The blank control was use water for the same steps.
Minimal bactericidal concentration (MBC) assay
Microwell dilution performed in sterile 96-well microplate was used to determine the MBC values of herbal medicine extracts against test bacterium according to22 with some modification. 2-fold serial dilutions with sterile water to obtain a concentration range from 0.78-100mg/ml. 100μl of 2-fold serial dilutions, and 100μl of inoculum (about 1×105 CFU) were added into each micro well of the microplate. The final volume in each well was 200μl. Water and methanol was used as a negative and a positive control (22.7 ng/ml-10μg/ml), respectively. Then it was incubated at 37°C for 24 hr for Staphylococcus aureus BCRC 10780, and 48 hr for Propionibacterium acnes BCRC 16147, and 10723, respectively. The concentration of hot water extracts with no macroscopically visible turbidity was taken as MBC. The MBC values were defined as the lowest concentration of the hot water extracts to bactericidal the growth of bacteria strains.
Cell culture
Human immortalized keratinocytes (HaCa T cells) were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, 100µ/ml penicillin-streptomycin. The cells were cultured in a humidified incubator at 37°C and 5% CO2. For most experiments, cells reaching a 90%~95% of confluency.23
Activity screening of different carbon and nitrogen source
The preliminary study was investigated for screening the effect of different carbon and nitrogen source on the antioxidant ability and inhibition of tyrosinase activity. Table 1 shows that there were various activity of supplemented with different carbon and nitrogen source in Monascus fermented rice extracts. This table include DPPH radical scavenging ability, reducing power and inhibition of tyrosinase activity, these sample was used water or methanol extract of Monascus rice, it added 2% different carbon source include glucose, glycerol, trehalose, maltose, citric acid; nitrogen sources include KNO3 or MSG before fermented. These results of measuring the antioxidant activity were that water extracts better than methanol extracts. It will influence the experimental result to add the carbon or nitrogen source, especially add KNO3 or MSG both in water and methanol extract. If regard added MRW and MRM as standard, these samples the majority will be higher than not added the carbon nitrogen source. DPPH radical scavenging, MRW and MRM add 2% KNO3 or MSG can increase 3.46, 4.81, 4.44 and 5.66 fold ability, respective. Reducing power, added KNO3 and MSG can increase 0.5, 1.1, 0.2 and 0.5-fold ability, respectively.
Sample |
Antioxidant ability |
||
DPPH radical scavenging ability (%) |
Reducing power (A700) |
Inhibition of tyrosinase activity (%) |
|
MRW |
9.09±0.12 |
0.41± 0.01 |
16.59±3.39 |
MRW+glucose |
6.32±0.49 |
0.58±0.02 |
21.29±1.03 |
MRW+glycerol |
5.88±0.28 |
0.45±0.01 |
19.88±2.79 |
MRW+trehalose |
2.14±0.72 |
0.47±0.01 |
22.45±3.42 |
MRW+maltose |
3.09±0.63 |
0.44±0.03 |
33.48±1.81 |
MRW+maltodextrin |
3.83±0.11 |
0.58±0.10 |
49.30±1.47 |
MRW+citric acid |
1.02±0.32 |
0.32±0.01 |
24.85±1.61 |
MRW+KNO3(MRWK) |
40.58±1.15 |
0.60±0.03 |
51.66±1.07 |
MRW+MSG (MRWM) |
52.82±4.26 |
0.85±0.08 |
69.76±1.44 |
MRM |
4.65±0.26 |
0.46±0.02 |
19.94±0.72 |
MRM+glucose |
4.22±0.20 |
0.46±0.03 |
15.02±3.08 |
MRM+glycerol |
3.39±0.16 |
0.40±0.06 |
6.41±0.19 |
MRM+trehalose |
1.76±0.54 |
0.41±0.01 |
18.65±2.22 |
MRM+maltose |
1.43±0.84 |
0.41±0.01 |
21.78±1.71 |
MRM+maltodextrin |
2.19±0.35 |
0.40±0.01 |
23.30±1.35 |
MRM+citric acid |
1.20±0.80 |
0.30±0.02 |
11.48±1.07 |
MRM+KNO3 (MRMK) |
25.30±3.85 |
0.58±0.01 |
33.76±2.63 |
MRM+MSG(MRMM) |
30.98±2.40 |
0.69±0.06 |
61.01±2.87 |
Table 1 Antioxidant ability and Inhibition of tyrosinase activity from various extracts Monascus rice mixture supplemented with 2% extra carbon or nitrogen source
Each value is expressed as mean±standard deviation (n=3). Sample concentrate of DPPH radical scavenging ability, 20 mg/ml; Reducing power, 2 mg/ml and inhibition of tyrosinase activity, 50 mg/ml. Monascus rice water extracts, MRW; Monascus rice methanol extracts, MRM.
The inhibitory effect of several copper-chelating agents on the activity of tyrosine by the tyrosinase was also tested.24,25 Among the several copper chelators including well-known tyrosinase inhibitors, such as kojic acid, MRWM and MRMM have shown the most significant inhibitory effect on the activity of tyrosinase. At 50 mg/ml, the concentration for the inhibition of tyrosinase activity were as abilities of MRW, MRWK, MRWM, MRM, MRMK and MRMM were 16.56, 51.66, 69.76, 19.94, 33.76 and 61.01%, respectively.
Add nitrogen source increase the anti-oxidant activity result rise, and anti-oxidant mechanism, which it was prevent aging and function protect skin.26‒29 In our research, the Monascus rice ferment of 5, 10 and 15 days then dried extract, discovery ferment 10 day extracts more high than 5 and 15 days extracts of anti-oxidant result and pigment content are determined too (data not shown). While fermenting, detect the change of pH value, only add citric acid change to reduce pH value, add other carbon, nitrogen source were no influence. This show Monascus rice add carbon, nitrogen source which anti-bacteria ability not influence by pH value change, Monascus rice ferment metabolite inhibit bacteria ability probably.30 Nitrogen limitation induced a switch of metabolic flux from glycolysis to the tricarboxylic acid (TCA) cycle for maintaining cellular energy homeostasis, resulting in repression of the metabolic shift of the polyketide biosynthesis pathway for red pigment production.31 Then our research will be aimed the Monascus fermented 10 day rice added KNO3 or MSG extracts research further in the following.
Extraction yield
Using water and methanol as the extract, the yields were in a descending order of MRWM (Monascus rice+MSG water extract)>MRWK (Monascus rice+KNO3 water extract)>MRMM (Monascus rice+MSG methanol extract)>MRW (Monascus rice water extract)>MRMK (Monascus rice+KNO3 methanol extract)>MRM (Monascus rice methanol extract) shows in Table 2. The higher yields of MRWM, MRWK and MRMM were mainly due to the fact that after the colonization of fungal growth.
Sample |
Extraction yield (g/100g) |
MRW |
21.72 |
MRWK |
26.82 |
MRWM |
29.66 |
MRM |
13.02 |
MRMK |
20.16 |
MRMM |
24.35 |
Table 2 Extraction yield of water and methanol extracts from various M. purpureus fermented rice products
Sample extracted from dried materials (100g). MRM, Monascus rice water extracts; MRW, Monascus rice methanol extracts. K and M mean add KNO3 and MSG.
Pigments produced from Monascus rice extracts
Table 3 shows that there was pigments concentration of supplemented with different carbon and nitrogen source in Monascus fermented rice extracts. Combined pigments of total yellow (400nm), orange (470nm), and red (500 nm) pigments because the yellow pigments (monascin and ankaflavin) and red pigments (monascoramine and rubropunctamine) are derived from the orange ones: monascorubrin and rubropunctatin.32 Monascus pigment can be prevents AAP-induced liver toxicity by both antioxidant action and the inhibition of AAP metabolism.33 It is relevant with antibacterial activities.34 Pigment yield increase by added nitrogen KNO3 or MSG. Added of MSG pigment yield more add KNO3 and un-added nitrogen. It may increase the anti-oxidant ability and inhibit bacteria activity by added MSG.
Monascus extracts |
Absorbance |
||
Yellow pigment (400nm) |
Orange pigment (470nm) |
Red pigment (500nm) |
|
MRW |
0.093 |
0.029 |
0.016 |
MRWK |
0.351 |
0.122 |
0.078 |
MRWM |
0.428 |
0.157 |
0.097 |
MRM |
0.155 |
0.05 |
0.028 |
MRMK |
0.372 |
0.144 |
0.104 |
MRMM |
0.551 |
0.252 |
0.186 |
Table 3 Different Pigments concentration of water and methanolic extracts from various Monascus rice products
MRM, Monascus rice water extracts; MRW, Monascus rice methanol extracts. K and M mean add KNO3 and MSG.
Determination of total phenolic and kojic acid content
Total phenolic content was expressed as gallic acid equivalents per gram of Monascus rice extract. The results showed in Table 1. Kojic acid is widely used as a food additive for preventing enzymatic browning, and in cosmetic preparations as a skin-lightening or bleaching agent.35 Kojic acid was tested in the agar diffusion test against twenty five dermatophytic fungi.36 The kojic acid has efficiency of whitening, antioxidant and antibacterial. Content of total phenol with antioxidant and antibacterial were relevant. The results showed that, in general, the stronger the antioxidant and tyrosinase inhibitory activities of these extracts, the higher the phenolic and kojic acid content. Monascus rice fermented added nitrogen source KNO3 or MSG have more total phenols and kojic acid content. Phenolic derivatives are structurally similar to the melanin precursor tyrosine, and therefore tyrosinase was originally implicated as a mediator of cytotoxicity, Phenolic compounds may be used as depigmenting agents and because they have a similar chemical structure to tyrosine, the substrate of tyrosinase.37 Kojic acid and total phenols content, add nitrogen source can increase 0.89~0.54; 1.24~2.26 fold, respectively. Inhibition tyrosinase activity also can increase 0.59~1.55 fold ability (Table 1). This show kojic acid and phenol can influence inhibition of tyrosinase activity increase ability. Thus, phenolics present in the extracts may play a major role in producing the results we obtained with the present studies.
Sample |
Total phenols (mg/g) |
Kojic acid (mg/g) |
MRW |
10.91±0.16* |
12.92±1.38 |
MRWK |
14.38±0.31 |
49.61±1.68 |
MRWM |
16.18±0.39 |
61.90±1.48 |
MRM |
11.42±0.24* |
20.25±0.62 |
MRMK |
14.04±0.52 |
39.33±0.72* |
MRMM |
14.70±0.34 |
44.05±1.49 |
Table 4 Contents of total phenols and kojic acid (mg/g) of water and methanolic extracts from various Monascus rice products
MRM, Monascus rice water extracts; MRW, Monascus rice methanol extracts. K and M mean add KNO3 and MSG. Each value is expressed as mean±standard deviation (n=3).
*Means with different letters within a column are significantly different (p<0.05)
Antibacterial activity
The antibacterial activities of Monascus rice extract were further evaluated by determining the minimum bactericidal concentration (MBC), which is the lowest concentration yielding on growth. The MBC was determined using a two-fold serial dilution method. Table 3 shows the in vitro growth inhibitory activity of Monascus rice extracts against clinical isolates of S. aureus and P. acnes. Both extracts inhibited the growth of S. aureus and P. acnes at concentrations between concentration of 1.56 to 50mg/ml. Inhibited the growth of Staphylococcus aureus BCRC 10780 at 6.25mg/ml of MRWK, MRWM, MRMK and MRMM, but at 50mg/ml of MRW and MRM. Inhibited Propionibacterium acnes BCRC 10723 was MRMM and MRWM at 3.13mg/ml; MRW, MRWK and MRMK at 6.25mg/ml; MRM at 25mg/ml. Inhibited P. acnes BCRC 16147 exhibit water extract at 3.13mg/ml and methanol extract 1.56mg/ml. Furthermore, the MIC of those extracts was 0.78mg/ml (data not shown). The difference between MIC and MBC has been established as an index of the bactericidal activity of antibiotics. 38) That orange monascus pigment has a weak antimicrobial activity whereas the red pigment has little or no activity. A change in the oxygen part of orange pigment to a nitrogenous compound (amino acid) causes a color change to red.39 (Table 5).
Sample |
S. aureus BCRC 10780 |
P. acnes BCRC 10723 |
P. acnes BCRC 16147 |
MRW |
50 |
6.25 |
3.13 |
MRWK |
6.25 |
6.25 |
3.13 |
MRWM |
6.25 |
3.13 |
3.13 |
MRM |
50 |
25 |
1.56 |
MRMK |
6.25 |
6.25 |
1.56 |
MRMM |
6.25 |
3.13 |
1.56 |
Table 5 Minimum bactericidal concentration (mg/ml) of water and methanol extracts from various Monascus rice products against epimeris pathogenic bacteria
Results are the mean of MBC values followed by the standard deviation. Each value is expressed as mean±standard deviation (n=3).
DPPH Scavenging ability
Figure 1 shows the effects of various extracts of M. purpureus on DPPH radical scavenging activity. At 100mg/ml, DPPH radical scavenged whereas abilities of MRW, MRWK, MRWM, MRM, MRMK and MRMM were 69.18, 78.81, 84.05, 62.06, 75.45 and 77.33%, respectively. The IC50, the concentration required for the inhibition of DPPH radical scavenging activity, was obtained from the figure were 13.46, 8.81, 8.79, 78.04, 37.07 and 25.55mg/ml, respectively. The inhibition of the MRWK and MRWM was nearly. The results imply that these active extracts may contain constituents with strong proton-donating abilities.40
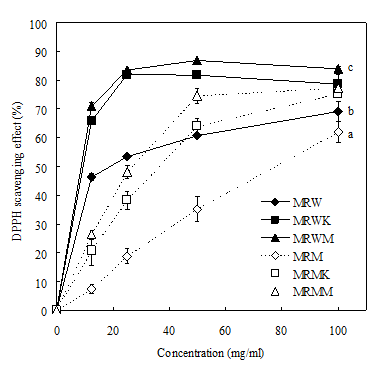
Figure 1 Scavenging ability of water and methanolic extracts from various Monascus rice products on 1,1-diphenyl-2-picrylhydrazyl radicals. Each value is expressed as mean±standard deviation (n=3). MRM, Monascus rice water extracts; MRW, Monascus rice methanol extracts.
Reducing power
Figure 2 shows the reducing powers of the M. purpureus on DPPH radical scavenging activity. At 1 mg/ml the reducing power of MRW, MRWK, MRWM, MRM, MRMK and MRMM were 0.17, 0.18, 0.23, 0.18, 0.16 and 0.20, respectively.
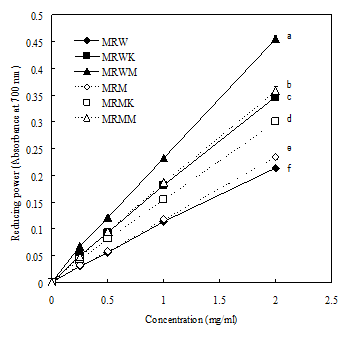
Figure 2 Reducing power of water and methanol extracts from various Monascus rice products. Each value is expressed as mean±standard deviation (n=3).
Inhibits UV-induced keratinocyte death
To determine the protective effects of Monascus rice extracts on human keratinocytes, we performed cell viability. Figure 3 shows MTT assay that cell viability of keratinocytes HaCa T cells treated with 500 ng/ml concentrations of various Monascus rice extracts, EGCG, catechin, vitamin C and kojic acid for 24 hr and then further incubated for 9 hr after UV irradiation. The viability ratio against UV irradiation was 42.32%, after pretreatment with EGCG, catechin, vitamin C, kojic acid, MRW, MRWK, MRWM, MRM, MRMK and MRMM were 64.13, 69.45, 64.00, 74.18, 57.50, 63.70, 64.93, 54.55, 57.68 and 63.37%, respectively. Relative with UV control, cell viability increase 75.27% (kojic acid), Both water and methanol Monascus rice extracts exhibit added MSG have best cell viability then other, were relative to increase 53.40% and 49.72%, respectively. This result shows that has ability of inhibits UV-induced keratinocyte death of Monascus rice extracts. Added the nitrogen source will increase protect ability.
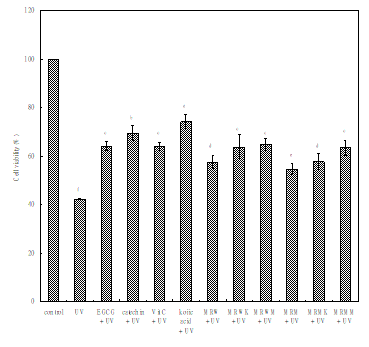
Figure 3 Inhibition UV-induced HaCaT cell desth. Keratinocytes pretreated with the indicated concentrations (500ng/ml) of water and methanolic extracts from various Monascus rice products were exposed to UV irradiation (52.1 J/m2) after kept at 37°C for 10min. Then, all cells were further incubated at 37°C for 9h. Cell viability ratio was evaluated using the MTT method. Results are expressed as percentage of control and are mean±S.E. (n=3).
In the present study, selected different carbon and nitrogen source in Monascus fermented rice extracts were investigated for potential effectiveness as protect skin, inhibit skin disease bacterial, skin whitening agents and inhibition UV-induced kerationcytes. Extracts of added MSG in water extract preparations were shown to be potent antioxidant activity on reduce power and DPPH scavenging ability. Kojic acid content and Inhibition of tyrosinase activity suppress ability in direct radio. Antibacterial ability, though it is methanol extracts ability higher than water extracts, but still was added MSG most high. This shows that adding MSG can increase antibacterial activity. Monascus rice extracts added MSG exhibit have best protect cell viability. Monascus rice ferment extracts added nitrogen source especially MSG may prove to have considerable value as cosmetic additives in the future. In fact, perhaps a lot of other effective composition still needs confirming in the fermented Monascus.
AS is fellow researcher of CNPq (Conselho Nacional do Desenvolvimento Científico e Tecnológico, Brazil).
None.

©2019 Lin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.