MOJ
eISSN: 2381-182X


Research Article Volume 2 Issue 5
Food Technology Division, Bhabha Atomic Research Centre, India
Correspondence: Satyendra Gautam, Head, Food Science & Safety Section, Food Technology Division, Bhabha Atomic Research Centre, Mumbai-400 085, India, Tel 022-25595379, Fax +91-22-25505151
Received: April 28, 2016 | Published: August 8, 2016
Citation: Verma J, Gautam S. Antimutagenic potential of date palm (phoenix dactylifera) fruit aqueous extract in suppressing induced mutagenesis and purification of its bioactive constituent. MOJ Food Process Technol. 2016;2(5):179-185. DOI: 10.15406/mojfpt.2016.02.00053
Date palm fruit aqueous extract was found to display strong antimutagenic activity against ultraviolet (UV) radiation, and mitomycin C induced mutagenesis, when analysed using E. coli RNA polymerase β based rifampicin resistance assay. However, the same aqueous extract did not show any significant antimutagenesis against ethyl methane sulfonate (EMS). Hence to further study the antimutagenic behaviour, the aqueous extract was purified to get the bioactive compound. Its 40% methanolic fraction (rich in phenolics) which displayed strong antimutagenicity against mitomycin C was further purified by HPLC and most potent peak P8 was obtained at 43min retention time. When E. coli cells were exposed to mitomycin C in the presence of this P8 compound, it showed differential regulation of some proteins, as observed in SDS-PAGE. Further, the study was extended to mammalian system using TK6 human lymphoblast cell line, where the compound P8 was found to display 40% antimutagenicity against mitomycin C induced mutation at thymidine kinase 1 (tk1) locus. Thus the findings indicate that purified bioactive P8 from date palm has the capacity to combat induced mutagenesis across the system indicating its nutraceutical potential.
Keywords: date palm, antimutagenicity, hplc, mitomycin C, UV, TK6 +/-
Due to health consciousness, consumption of fruits and vegetables has significantly increased in recent years particularly because of their recognition as one of the healthiest natural foods and this has also resulted in enhanced research interest in this area. With increase in the incidence of lifestyle associated chronic diseases, such research findings have immense significance. Mutagenic contaminations are one of the major causes of diseases like neoplastic induction. Therefore understanding about the nature of constituents present in food having potential to control or reduce such incidence of mutations and its mechanism of action is quite relevant and significant. Phytochemicals from fruits and vegetables have gained increased interest due to their health protective attributes including antioxidant and antimutagenic properties. The fruit of date palm is highly delicious and nutritious1 and is consumed as a dietary staple in many parts of the world.2 Date fruit can be consumed at any of the three stages of maturity such as khalal (fresh, hard ripe, color stage), rutab (crisp to succulent or ripe stage), or tamr (soft pliable, full ripe stage). Fresh dates are considered as nutritionally superior and exceptionally delicious compared to dry one.1,3 Date palm fruit has been reported to possess many classes of bioactive compounds such as carotenoids, polyphenols especially phenolic acids, lignans, and flavonoids (including isoflavones), tannins, and sterols.2,4 Several studies have reported good correlation between antioxidant capacity and the polyphenolic content of date fruit.1,5-9 The chemo preventive potential of date fruit against chemical carcinogens has been reported in Salmonella tester strains. Fresh aqueous extract of date fruit was found to inhibit benzo(a)pyrene-induced mutagenicity in Ames assay in strains TA-98 and TA-100 in a dose dependent manner.5 Most of the studies related to health protective properties of date palm fruit have remained confined to its crude aqueous extract, and characterization of the bioactive compounds contributing to antimutagenicity is still lacking. The current study deals with the evaluation of antimutagenic potential of date fruit aqueous extract against different direct acting mutagens and further to purify the bioactive compound contributing to this antimutagenicity.
Procurement of date fruit and preparation of aqueous extract
Fresh Barhee date fruit samples at their khalal stage (fresh, hard ripe, color stage) were procured from local market in Mumbai, India. The fruits were washed with portable water. The pulp including peel of fruits were ground with mortar and pestle in liquid nitrogen and stored at -70°C till further use. The ground date fruit powder was mixed with water to maximum solubility (0.5 g/ml) for 10 min at 37°C. The suspension was then centrifuged at 8000g, for 10 min and supernatant having aqueous extract was stored at 4°C and used for further analysis.
Chemicals
Luria-Bertini (LB) medium, rifampicin, RPMI (Roswell Park Memorial Institute) 1640 medium and fetal bovine serum (FBS) were procured from HiMedia Laboratories Pvt. Ltd., Mumbai (India). Cytidine, thymidine, trifluorothymidine, hypoxanthine, aminopterin, L-glutamine, Magnesium sulfate, Folin Ciocalteu’s phenol reagent, gallic acid, acetonitrile, ethyl methanesulfonate, mitomycin C and methanol were procured from Sigma Chemical Co. (St. Louis, MO, USA). Formic Acid and hydrochloric acid were procured from SD Fine Chemical (India). Milli-Q water was obtained using a Millipore system. Pen-Strep (Penicillin and streptomycin) was procured from Gibco Life Technologies, USA.
Bacterial strain and Cell line
Escherichia coli strain MG1655 (F- λ- ilvG- rfb-50 rph-1) was gifted by Prof. M. Z. Humayun, Rutgers New Jersey Medical School, NJ, USA. Thymidine kinase (TK6) human lymphoblast cell line (genotype: tk+/-) was obtained from National Centre for Cell Sciences (NCCS), Pune, India.
Estimation of antimutagenic potential
The antimutagenic activity was assessed using rifampicin resistance (RifSàRifR) assay as described earlier.10
Ultraviolet (UV) induced mutagenesis: Overnight grown E. coli MG1655 was subcultured in Luria-Bertini medium (25 ml, 1:50 dilution) and grown at 37°C for 3 h at 125 rpm in an orbital shaker. The cells were centrifuged (8000g for 10 min) and pellet was resuspended in sterile MgSO4 (0.2 M). An aliquot (50 µl) of cell suspension was exposed to UV (254 nm; lamp power, 8W; Intensity, 220 μW/cm2) for 10, 15 and 20 s, in presence and absence of the date palm aqueous extract. It was then inoculated in 1 ml LB in 3 replicates for overnight at 37°C. The culture was serially diluted using sterile saline (0.85%) and spread plated on LB-agar-rifampicin (100 µg/ml) plates (for scoring RifR mutants) and on LB-agar plates (for enumerating viable cells) and incubated at 37°C for 24 h. Mutation frequency was determined by calculating the ratio of the total number of RifR cells per ml to the total number of viable cells per ml.
Mitomycin C induced mutagenesis: LB-grown mid-log phase cells (1ml) were centrifuged and the pellet was resuspended in sterile LB medium. The suspension was incubated with mitomycin C (3.3 µM, 6.3 µM, 10µM) in the absence (control) as well as presence of the date fruit aqueous extract at 37°C for 1 h at 125 rpm. The cells were centrifuged and pellet was washed twice with LB and resuspended in 2ml of the same. The culture was further grown for 4 h at 37°C and its appropriate dilutions prepared in sterile saline (0.85%) were spread plated on selective plates and mutation frequency was calculated as described above.
EMS induced mutagenesis: LB-grown mid-log phase cells (1ml) were centrifuged at 8000 g for 10 min and the pellet was resuspended in LB medium. Antimutagenic assay was performed by incubating cell suspension with EMS (33.25, 66.5 and 133 mM), in the presence as well as absence of the fruit extract and incubating at 37°C for 45 min at 125 rpm. The cells were centrifuged and pellet was washed twice with LB and resuspended in 1 ml of the same. An aliquot (50 µl) of the cell suspension was inoculated in LB broth (1 ml), grown overnight at 37°C on a rotary shaker (125 rpm) and its proper dilutions were spread plated and mutation frequency was calculated as described above.
Total phenolic content
The total phenolic content was determined by Folin-Ciocalteu method.11 An aliquot (250 μl) of date palm aqueous extract (0.5 g/ml) was mixed with 7.75 ml of water and 0.2N Folin-Ciocalteu reagent (500 μl). The solution was vortexed and kept at ambient temperature for 5 min. Later 1.5 ml of 20% sodium carbonate solution was added and kept for 20 min at 40°C. The solution was then ice-cooled and absorbance was measured at 755 nm using a spectrophotometer (Shimadzu, UV-1700, UV-Vis Spectrophotometer). The total phenolic content was estimated by comparing with a standard curve prepared using gallic acid (0-50 μg/ml) and expressed as mg GAE/100g fresh weight of date palm fruit.
Solid phase extraction (SPE)
A C-18 Sep-Pak column was preconditioned with 24 ml methanol and then equilibrated with acidified water (0.01% v/v conc. HCl). The date palm aqueous extract was acidified with 0.01% v/v conc. HCl. Its 2 ml volume was loaded on to the column, which was later washed with acidified water. Phenolics were eluted with increasing concentration (20-80%) of methanol.
High performance liquid chromatography
The SPE-purified methanolic extract was further purified using reverse phase HPLC system (Ultimate 3000, Dionex, Sunnyvale, California, U.S.A.) equipped with C-18 analytical column (4.6 x 250 mm, Acclaim 120 and pore size 5 μm) having variable wavelength detector (VWD-3000) and Chromeleon 6.8 software. Analytical separation was carried out in a gradient system (eluent A= water/formic acid v/v 99:1, eluent B= absolute methanol). The eluent gradient used was as follows: 0-5 min, 5-10% B; 5-10 min, 10-15% B; 10-65 min, 15-40% B; 65-70 min, 40-100% B; 70-90 min 100-5% B. An aliquot (20µl) was injected and HPLC was performed at the flow rate of 1 ml/min. The individual peaks were collected, vaccum dried, concentration was normalised to that from 0.5 g/ml of aqueous extract, dissolved in milli-Q water and tested for antimutagenic activity.
SDS-polyacylamide gel electrophoresis
LB-grown mid-log phase cells were centrifuged and the pellet was resuspended in LB medium. The cells were treated with mitomycin C (6.3 μM) in the absence and presence of purified compound, and grown for 4 h. The cells were washed with PBS (phosphate buffer saline, 10 mM, pH 7.4), suspended in milli-Q water, and lysed by mixing with lysis buffer (100mM Tris-HCl, pH 6.8; 4% SDS; 20% glycerol; 0.02% bromophenol blue and 200mM β-mercaptoethanol) and further heating at 95°C for 10 min. An aliquot of the supernatant equivalent to 25 μg protein was run on a 12% (W/V) SDS polyacrylamide gel, which was later stained overnight with coomassie Brilliant blue (CBB) and then destained to visualize protein bands. Band intensity of the protein gel was compared using Image J software version 1.48v (http://imagej.nih.gov/ij).
TK6 gene mutation assay
The thymidine kinase gene mutation assay exploits the heterozygous thymidine kinase (tk) gene locus of TK6 human lymphoblast cell line, which undergoes forward mutation (tk+/- àtk-/-) upon mutagen exposure. These mutant cells lacking thymidine kinase activity survive the cytotoxic effect of trifluorothymidine (TFT), which is a pyrimdine analogue and blocks the DNA synthesis after phosphorylation by thymidine kinase.
The assay was performed as described earlier,12 where the TK6 cells were purged for the spontaneous mutants by growing in medium containing CHAT (10-5 M cytidine, 2X10-4 M hypoxanthine, 2X10-7 M aminopterin and 1.75X10-5 M thymidine). Aminopterin in the medium blocks the denovo synthesis of nucleotides, thus only tk+/- cells can survive as they can utilize the exogenous thymidine in the medium. After two days the medium was replaced with CHT medium (without aminopterin), and grown for further 1 day. These exponentially growing cells at a density of ~2X105 cells/ml, were treated with mitomycin C (1 µM) and grown further for 4 h. Higher concentration of mitomycin C was found to be toxic to the cells. Treatment was carried out at 37°C in a humidified CO2 incubator. After the treatment, cells were washed twice with the phosphate buffer saline (10 mM, pH 7.4). The cultures were grown for further 2 days in non-selective conditions for expression of tk-/- phenotype. After that ~2X105 cells from each treatment were grown in selective medium containing 5 μg/ml TFT for 3 days at 37°C in a 5% CO2 incubator to score tk-/- cells. Similar experiments were performed in the presence of the purified bioactive from date palm for the evaluation of its antimutagenic potential against induced mutagenesis. The mutant tk-/- cells which survived the cytotoxic effect of TFT formed cell aggregates in the suspension cultures thereby increasing the total number of viable cells. The cellular aggregates were visualized and photographed using an inverted compound microscope under 10X objective (Carl Zeiss AxioVert.A1, Germany equipped with Tucsen ISH500 camera). The total number of live cells was counted by trypan blue exclusion test using haemocytometer where the dye stains the dead cells and live cells remain unstained due to their intact cell membrane. The result was expressed as the relative number of tk-/- mutants per ~105 seeded cells, where
Statistical analysis
All experiments were conducted in three replicates, means and standard errors were calculated taking all the readings into consideration. Statistical analysis was performed using Graph Pad Prism version 6.05 for Windows (Graph Pad Software, San Diego, CA). One-way ANOVA was performed to ascertain the significance of differences in mean values at P ≤ 0.05. Chromatographic and spectral analysis were performed in three independent sets and data from a representative set has been shown.
Date palm aqueous extract displayed variable antimutagenicity against different mutagens
To investigate the antimutagenic potential of date palm its aqueous extract was assayed against different direct acting mutagens namely ultraviolet radiation (λ 254 nm), mitomycin C and EMS using E. coli RNA polymerase β (rpoB)-based rifampicin resistance (RifSàRifR) assay upon mutagenic challenge. This bacterial assay system monitors acquisition of rifampicin resistance, which resulted from mutation in the β-subunit of RNA polymerase. The spontaneous mutation frequency in this assay is extremely low due to stringent regulation of the hot spots of mutation in rpoBgene. Ultraviolet (UV) radiation is known to damage the DNA due to the formation of pyrimidine dimers and 6-4 photoproducts.13 UV treated E. coli MG1655 cells showed mutation frequency of 223x10-8 cells at exposure time of 10 s, 318x10-8 cells at 15 s and 825x10-8 cells at 20 s. In presence of 0.5 g/ml of date palm aqueous extract mutation frequency was reduced by 45-50% in all these UV exposed samples (Figure 1A). Although a UV dose dependent increase in RifR mutation frequency was observed in this assay, however no such effect was observed in antimutagenicity displayed by the aqueous extract at the concentration used. Here in all the cases around 50% of antimutagenicity was observed. Another mutagen mitomycin C which leads to DNA intercalation and adduct formation14 was able to induce mutation frequency to 130x10-8 cells at 3.3 µM, 205x10-8 cells at 6.3 µM and 155x10-8 cells at 10 µM. The presence of 0.5 g/ml date palm aqueous extract was able to reduce mutation frequency by 74± 6% in 3.3 μM mitomycin C treated sample, 53 ± 8% in6.3 μM sample and 36 ± 5% in 10 μM mitomycin C treated sample (Figure 1B). Here in contrast to UV, with increase in the concentration of mitomycin C decrease in antimutagenicity by date palm aqueous extract was observed. Ethyl methane sulfonate (EMS) does ethylation of guanine in DNA causing GCàAT transitions.15 EMS treatment was found to induce RifR mutation frequency to 150x10-8 cells at 33.25 mM, 448x10-8 at 66.5 mM and 543x10-8 cells at 133 mM. However, no reduction in mutation frequency was observed at any of these concentrations. Among the two chemical mutagens used mitomycin C was found to be highly potent mutagen compared to EMS. Previous studies too have shown such variable antimutagenicity where date palm aqueous extract showed antimutagenicity against Benzo(a)pyrene, but not against sodium azide in Ames test.5 The dietary antimutagens have been reported to work through different mechanisms such as modulation of DNA replication and repair pathways, inhibiting the DNA binding of mutagens, prevention of nitrosylation, and protection against oxidative damage.16 The observed antimutagenicity of date palm aqueous extract may be possibly due to one of these mechanisms. Although ultraviolet radiation is a potent mutagen, there is the possibility of its shielding by the compound in the extract putting question on the observed antimutagenic potential of the extract. Therefore mitomycin C which works through similar mechanism like UV, was selected for further experiments. The higher conc. (10 µM) of mitomycin C was found to be toxic to cells, resulting in reduced viability and preferential killing of mutant cells as indicated by reduced mutation frequency at this concentration compared to lower concentrations. Therefore 6.3 µM concentration of mitomycin C was selected for further studies.
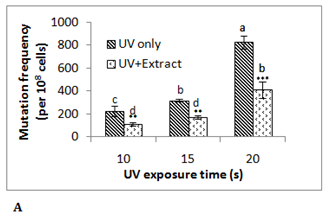
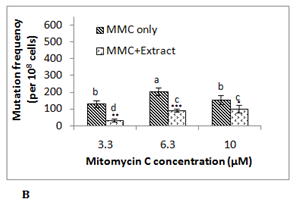
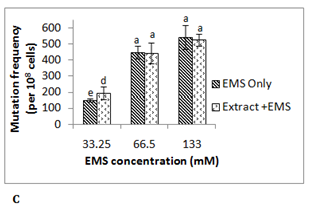
Phenolic content in date palm fruit
In earlier reports phenolics from different fruits such as strawberries, raspberries, grapes and walnuts were found to display strong antimutagenicity.17 Hence during this study too the phenolics of date palm were assayed for this activity. Although the significant variation has been reported in the phenolics content of date palm fruit, fresh Barhee date fruit used in this study was found to be rich in phenolics (176 ± 4mg GAE/100g fresh weight). In Tunisian dates the total phenolic content has been reported to vary from 1.6 mg/100 g fresh weight to 448 mg /100 g fresh weight in different cultivars.8,18 In Bahrian date belonging to different geological location phenolic content ranged from 67-376 mg GAE/100 g fresh weight.19 In some reports very high phenolics content has been reported in date fruit such as in case of Algerian dates (955 ± 7 mg GAE /100g),20 Mauritanian dates(853 ± 5mg GAE /100g),11 and Chinese dates(586 ± 19 mg GAE/100 g). As observed in case of date fruit, phenolics content was also reported for many other fruits too, and there also it varied drastically such as sweetsop (405 ± 17 mg GAE/100 g), guava (194 ± 7 mg GAE/100 g), pomegranate (147 ± 0.04 mg GAE/100 g), Chinese wampee (116 ± 7 mg GAE/100 g), cherries (115 ± 5 mg GAE/100 g), and plum (sanhua) (102 ± 3 mg GAE/100 g).21
Methanolic extract rich in phenolics displayed strong antimutagenicity
Phenolics were extracted by performing methanolic extraction. To achieve better purification the aqueous extract was further extracted step-wise with solvents such as hexane, diethyl ether and chloroform to remove more non-polar impurities. Later this partially purified aqueous extract was further subjected to solid-phase extraction (SPE) using Sep-Pak column, and the phenolics were eluted with increasing concentrations of methanol (20-80%). Spectrophotometric analysis in UV region showed that majority of phenolics were present in 40% and 60% fractions. These fractions were vaccum dried, reconstituted in milli-Q water and further analysed for antimutagenic activity. The 40% methanolic fraction displayed 45% antimutagicity, which was 15% higher than 60% fraction (Figure 2). This indicates comparatively more polar nature of the bioactive compound responsible for observed antimutagenicity. Classes of bioactive phenolics reported from different sources in earlier studies and associated with antimutagenic activity are phenolic acids, hydrocinnamic acids, coumarins, stilbenes, anthraquinones, flavonoids, diarylheptanes, lignans, and lignins.17 Date fruit too has been reported as predominant source of benzoic or cinnamic acid derivatives, and also contained gallic, procatechuic, p-hydroxybenzoic, vanillic, caffeic, p-coumaric, and ferulic acids as major phenolic acids. In recent studies the presence of sinapic acid, 5-o-caffeoylshikimic acid and its isomers have been reported in seven varieties of ripe Algerian date fruit.6 Presence of chlorogenic and isochlorogenic acid have also been reported in date palm. In some other studies flavonoids and proanthocyanidines have also been reported in dates. Flavonoids reported in dates include proanthocyanidines especially procyanidines and flavonoid glycosides.6,22 However, anthocyanins have been reported to be present in extremely low amounts in dates.3
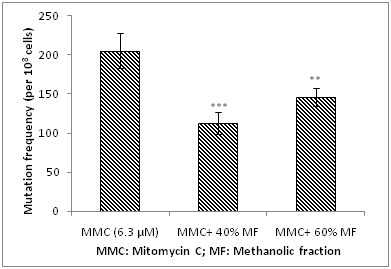
Figure 2 Analysis of antimutagenic potential of methanolic fraction of date palm fruit against mitomycin c-induced mutagenicity.
Statistical significance, *P<0.05; **P<0.01; ***P<0.001 with respect to control (MMC 6.3 μM).
HPLC peak at Rt 43 min displayed strongest antimutagenicity
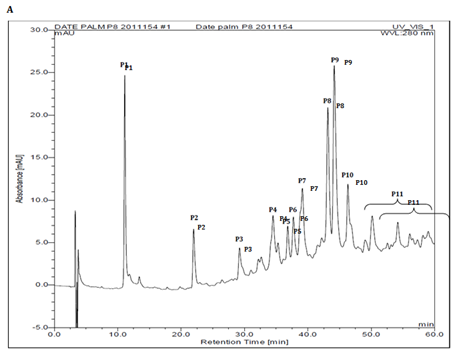
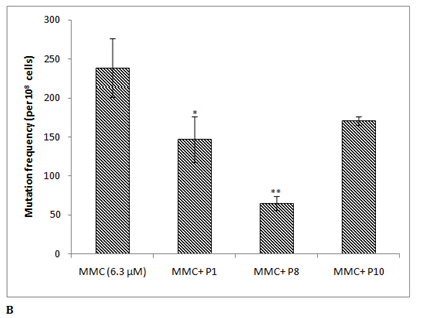
The phenolic profile of 40% methanolic fraction of date fruit showed 11 major peaks (P1-P11), when analysed in the methanol and water (1% formic acid) gradient (Figure 3A). These peaks were collected individually and analysed for antimutagenicity against mitomycin C. Out of these, three peaks P1 (Rt~7.5 min) , P8 (Rt~43 min) and P10 (Rt ~45 min) showed antimutagenic activity 38 ± 12%, 73 ± 4% and 28 ± 2% respectively (Figure 3B). The identification of the peak P8 showing highest antimutagenicity will be addressed in a separate study. Earlier the date palm phenolic profile has been reported in the acetonitrile and acidified water gradient system,20,23 but in this study methanol was used because the resolution of the peaks was found to be better compared to other reported systems (data not shown).
Plant phenolics and their role in antimutagenicity
Effect of caffeic acid and chlorogenic acid on mutagenicity was earlier studied using the Ames test. These compounds had inhibitory effects on the mutagenicity of Glu-P-2 (2-amino-dipyrido[1,2-a:3',2'-d]imidazole). Compounds analogous to caffeic acid, such as cinnamic acid, coumaric acid, and ferulic acid, also significantly decreased the mutagenicity of Glu-P-2.24 Ferguson and others25 have determined the abilities of (E)-ferulic acid, (E)-p-coumaric acid and (E,E)-5-5-dehydrodiferulic acid to protect against different types of mutation in a simple bacterial model. Birosova et al.26 examined the antimutagenic potential of caffeic, ferulic, as well as of another phenolic acids, on 3-(5-nitro-2-furyl) acrylic acid (5NFAA) and sodium azide mutagenicity. In the case of 5NFAA, the antimutagenic potency of tested compounds was in the order of gallic acid >ferulic acid >caffeic acid >syringic acid >vanillic acid.
P8 compound affected the expression profile of protein in E. coli cells
To further decipher the molecular events resulting in antimutagenicity displayed by P8 compound, the SDS-PAGE analysis of the E. coli MG1655 cells exposed to mitomycin C (6.3 μM) in the presence, as well as absence of this compound was performed. The protein band intensity showed the down-regulation of expression of some proteins (Band 7 and 9), and up-regulation of some other proteins (Band 8) as compared to the mitomycin C exposed cells in the absence of the purified compound (Figure 3C). This indicated that the P8 compound is acting at the molecular level which eventually resulted in the differential expression of protein in the cells. However knowing the exact nature of these proteins (up- or down-regulated) is part of further study and will certainly help in deciphering the exact molecular events taking place. Certain polyphenols have been reported earlier to show direct influence on the gene expression partially affecting the activity of DNA repair enzymes. Some of these include vanillin (VAN), cinnamaldehyde (CIN), coumarin and tannnic acid, which were reported to modify DNA replication and repair upon DNA mutation induced damage.17
P8 showed significant antimutagenic activity at tk locus in human lymphoblast cell line
The TK6 human lymphoblast cells are heterozygous at thymidine kinase (tk) locus, which after mutagenic exposure may become deficient in thymidine kinase due to mutation leading to tk+/- genotype à tk-/- genotype. The thymidine kinase proficient cells are sensitive to trifluorothymidine (TFT), where after incorporation thymidine kinase converts TFT to TFT-monophosphate which blocks the denovo synthesis of nucleotides, and blocks further cell division, however the mutant cells (tk-/-) become resistant to cytotoxic efffect of pyrimidine analogue TFT. Previous studies from Food Technology Division, BARC have shown that procyanidin from apple cv. display potent antimutagenic potential against EMS induced mutagenesis in TK6 cells.12 Therefore to further extrapolate our findings in mammalian system, TK6 gene mutation assay system was utilized. The HPLC purified peak P8 was analysed against mitomycin C induced mutagenicity in TK6 human lymphoblast cell line at tk locus. The induced mutants survived in the TFT containing media and formed cellular aggregates in suspension (Figure 4A). The relative number of induced mutants on exposure to mitomycin C (1 µm) was found to be ~3X104 (± 1X103) cells per ~105 seeded cells. The mitomycin C induced mutagenicity was reduced by 40% in presence of the purified peak P8 (Figure 4B). However there was no induced mutagenesis observed in P8 alone treated cells. This shows that the purified bioactive P8 from date palm has potential to reduce the induced mutagenic events in human cells, however the mechanism of antimutagenicity remains to be elucidated.
.png)
.png)
Figure 3A HPLC analysis of 40 % methanolic fraction of phenolic extract showing the peaks analysed.
Figure 3B HPLC analysis of 40 % methanolic fraction of phenolic extract showing the peaks analysed.
Figure 3C Protein expression profile: (i) SDS-PAGE (Lane L1: Control, Lane L2: P8 treated, Lane L3: MMC 6.3 μM treated, Lane L4: MMC+P8 treated and (ii) Relative band intensity.
Values are mean band intensity of two independent experiments ± SEM. Statistical significance, *P<0.05; **P<0.01; ***P<0.001, ****P<0.0001 with respect to control (untreated).
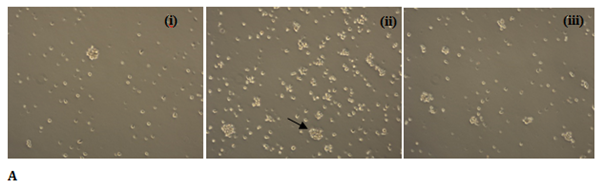
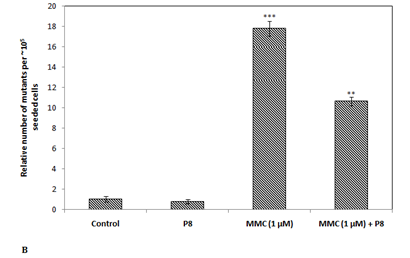
Figure 4 (A) Cellular aggregates (marked) are mutant (tk-/-) cells which survived in trifluorothymidine (TFT) containing media (i) Untreated control, (ii) MMC treated, and (iii) MMC+P8; (B) Antimutagenic potential of HPLC purified peak fraction P8 in thymidine kinase human lymphoblast cell (tk+/-) line.
Statistical significance, *P<0.05; **P<0.01; ***P<0.001 with respect to control (untreated).
In the current study the fresh Barhee date palm aqueous extract was found to have variable antimutagenicity against different mutagens. Its most potent bioactive compound was HPLC purified, which displayed significant antimutagenic activity against induced mutagenicity in different model systems such as bacteria and human lymphoblast cell line. The current findings thus indicate that date palm aqueous extract contains bioactive of immense antimutagenic potential establishing its role in development of nutraceutical having potential to combat induced mutagenesis.
The authors acknowledge Prof. M. Z. Humayun, Rutgers New Jersey Medical School, NJ, USA, for gifting E. coli MG1655 strain.
JV standardized the protocols, conducted the experiments and drafted the MS., SG conceptualized the idea, designed the experiments, and edited the MS. The authors also declare that there is no conflict of interest.

©2016 Verma, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.