MOJ
eISSN: 2381-182X


Research Article Volume 5 Issue 2
Department of Chemical Engineering, Regional University of Blumenau, Brazil
Correspondence: Fernanda Raquel Wust Schmitz, Department of Chemical Engineering, Regional University of Blumenau, São Paulo Street, 3250, Blumenau, SC, Brazil, Tel 55 47 32216065
Received: September 13, 2017 | Published: November 2, 2017
Citation: Schmitz FRW, Paulo IA, Carvalho LF, et al. Advanced methodology for analysis of changes in the storage lettuce surface. MOJ Food Process Technol. 2017;5(2):259-261. DOI: 10.15406/mojfpt.2017.05.00121
Several methods are used to evaluate behavior of vegetables, but there are still doubts about what is the best for each case. The scanning electron microscopy (SEM) has been used in studies to obtain knowledge about morphological characteristics of foods, especially foods with short shelf life and is constantly present in the diet of Brazilians. Based on this information, the scanning electron microscope was used to check the changes in different regions on the surface of curly lettuce leaves, during 14days refrigerated storage (4°C), in Styrofoam trays with polyvinyl chloride (PVC) film in relation to metabolic activities. In the studied regions were found stomata and monitoring the humidity loss process of samples (wilting), due to the breathing rate and transpiration through the images obtained by (SEM). In the first days of refrigerated storage the stomata were open, demonstrating that the lettuce was in the process of perspiration. Thus, it is concluded that the SEM methodology is able to demonstrate the lettuce behavior during the cooling period, showing the senescence process as wilting.
Keywords: scanning electron microscopy, lettuce, breath rate, transpiration
The feeding of the world’s population it is changing in the last years, becoming more targeted to wellness and prevention of diseases. In this context, many leafy vegetables are involved, like lettuce, one of the most consumed, rich in antioxidants like quercetin, kaempferol, luteolin and vitamin C, as also source of fiber that help to regulate the intestinal tract.1 Lettuce is found minimally processed to facilitate its preparation and consumption, it being offered in washed whole leaves; washed cut leaves; in packaging with modified atmosphere or only in packaging with films.2
To ensure the safety of food and their shelf life, besides packaging to protection against contamination by microorganisms, insects, rodents, physical stress and control of gas exchange and water steam, also is used refrigeration and high relative humidity.3,4 Vegetables shelf life and structural features are directly related to water steam loss rate that occurs by stomata, responsible for transpiration, or by cuticle, a wax layer found on leaf surface to avoid high water loss.5 Stomata are structures found in lower epidermis of ground plants to control the influence of the high sun incidence, decreasing excessive transpiration rate of vegetables.5 According to Kader,6 vegetables present different breath rates, lettuce leaves and cauliflower have breathe rate between 20 and 40 mg CO2.kg-1.h-1 (5°C). The minimum processing of vegetables may increase breath rate, causing undesirable changes in color, flavor, texture and nutritional quality of foods.6
Scanning electron microscopy (SEM) offers differentiated and advanced methodology for analysis the phenomena that occur in the micrometric and sub micrometer scale.7,8 The use of this equipment allows getting much information from different characteristics of the studied samples, like surface topography, composition and crystallography8 and their use is very common in the areas of biology, odontology, pharmacy, engineering, chemistry, metallurgic, physics, medicine and geology.9 Thus, this work present the use of scanning electron microscopy (SEM) for micrographic analysis throughout the shelf life of crispy lettuce samples minimally processed and stored in household refrigerator.
Plant materials
Crispy lettuces (Lactuca sativa L.), grown in hydroponics system in Blumenau/Santa Catarina, were collected in the morning (7 a.m.) and selected based on the characteristics relating to “harvest point to be commercialized”, like color pattern, size and absence of defects. Then, samples were transported in thermal boxes to Food Processing Laboratory, of the Department of Chemical Engineering, FURB, where the study was conducted.
Samples preparation
After the selection according to size, color and absence of defects, lettuce samples (14 samples) were placed in polyethylene expanded packaging with polyvinyl chloride (PVC) covered and stored in refrigerated incubator (B.O.D.) at 4°C ± 3°C and relative humidity of 70% ±10% for 14 days. In this period, temperature and relative humidity were monitored with Klimalogg Pró.
Scanning electron microscopy (SEM)
To verify changes on the surface of curly lettuce leaves, during 14 days of storage at 4°C, SEM (TESCAN, model VEGA 3 SEM) was used. This methodology use electron beams to explore the sample’s surface, as described by Dedavid et al.9 The equipment was used the sample vacuum metallizer (QUORUM, model Q150R ES) to apply a gold film (35 nm thick) in samples of 1 cm of diameter taken from the top leaf region and central lettuce leaf region and fixed with double sided tape in aluminum support , so that the surface of the lettuce could be visualized through SEM. After the sample was metalized, it was inserted in the SEM to capture the images with magnification of 200 times to verify sample’s surface.
The analysis allows to detect large number of stomata on the sample’s surface as seen in Figure 1, concerning to the first day of storage. Through the captured images it is possible to analyze the surface of the lettuce leaf on the harvest day, presenting smooth surface and the central rib without damage. The SEM images of the top and center samples of the leaf show that the structure of the leaf surface is more elongated, conferring with the studies of Lima.10 The presence of stomata was also detected, and these were open at the time of image capture.
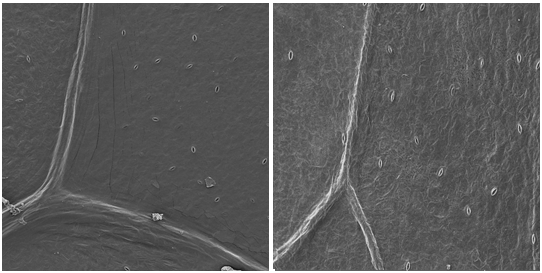
Figure 1 Micrographs of lettuce leaf on the first day of storage at 4°C; (a) top leaf region and (b) central leaf region, with magnification of 200 times.
The quantity of stomata found may be explained by growth conditions of plants environmental (like the availability of water in the environment) and storage of the minimally processed product.11,12 The presence of stomata is related to the environment in which the plant lives, and are positioned in the attempt to reduce the loss of water as steam, but have little taxonomy application.13 The study conducted by Takeuchi et al.14 demonstrated that how greater the number of stomata per unit of lettuce’s surface area, greater the chance of entrance of the cells of E. coli. O157:H7, influencing microbiological food safety. It may be prevented with good practices during vegetable handling and use of packaging.15
It was verified low number of stomata on the sixth day of storage as shown in Figure 2. As in the first day, the stomata were open, showing high transpiration (gain or loss of water) which is stomata function in plants, proportional to breath rate.16 On the sixth day, leaf’s surface had shriveled appearance, according17 this could be caused mainly by physicochemical changes such as the loss of water for the environment. As shown in Figure 3, lettuce leaves were very rough; this may be due to breath activity along the shelf life on the fourteenth day of storage.
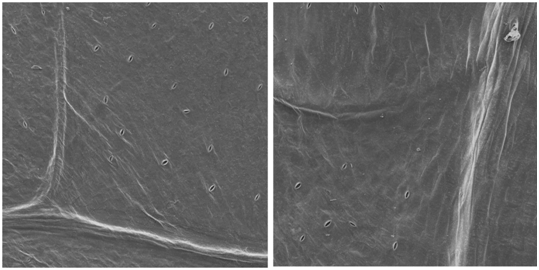
Figure 2 Micrographs of lettuce leaf on the sixth day of storage at 4°C; (a) top leaf region and (b) central leaf region, with magnification of 200 times.
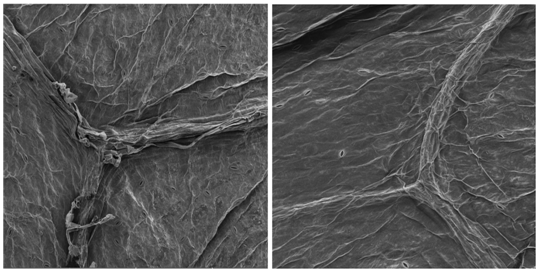
Figure 3 Micrographs of lettuce leaf on the fourteenth day of storage at 4°C; (a) top leaf region and (b) central leaf region, with magnification of 200 times.
A small number of stomata were observed in the last day of storage. According Fonseca SC et al.18 this is natural in vegetables in senescent phase, but that still allowing food’s water transfer to the environment, affecting directly in the vegetables sensory characteristics. According Galvão et al.17 the wilting process, that gives high roughness to the samples, is associated with water loss in vegetables, leading in weight loss and quality. These losses occur due to metabolic reactions that change product texture and the transpiration rate that minimizes the wilting process, which can be done with increased of relative humidity in the storage place, decreased of temperature, use of nutrients in the preharvest that influence in plant cell structure and others. Conti et al.19 verified that, depending on the growing region, climate and cultivar, there are more or less variation in the number of stomata, the crispy leaf variety has 6585 to 7279 stomata/cm² and butter variety has 4405 to 9487 stomata/cm².
The methodology used in this study had results that allowed observe and analyze sample’s surface with details of micro structural changes during the refrigerated storage period, mainly related to morphological characteristics. This method also allows analyzing the contaminating microorganisms present in lettuce leaves and through the generated images it is possible to determine microorganism’s types. Furthermore, SEM stands out when compared with others methodologies, because allows to obtain the estimate of the number of stomata by area and visual structural changes, that are directly related to the development stage of the vegetable, respective breath and transpiration rates.
We thank the Regional University of Blumenau (FURB) and the Foundation for Research and Innovation of the State of Santa Catarina (FAPESC) for allowing this research.
The author declares no conflict of interest.

©2017 Schmitz, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.