MOJ
eISSN: 2381-182X


Research Article Volume 6 Issue 3
College of Life Sciences, Dalian Minzu University, China
Correspondence: Aili Jiang, College of Life Sciences, Dalian Minzu University, Dalian 116600, China, Tel 13840853022, Tel 13591162665
Received: May 15, 2018 | Published: May 22, 2018
Citation: Jiang A, Gua S, Zhoua F, et al. Effect of the extraction of Perilla frutescens treatment on quality of fresh-cut red cabbage (Brassica oleracea). MOJ Food Process Technol. 2018;6(3):258-262. DOI: 10.15406/mojfpt.2018.06.00173
It is vulnerable for red cabbage to decline in quality after cutting, causing seriously financial losses. In this study, the fresh‒cut red cabbage was soaked with 50% Perilla frutescens extraction (50% extraction), 100% Perilla frutescens extraction (100% extraction) and distilled water for 5 min, respectively, to study the effects of Perilla frutescens extraction treatment on quality and antioxidant capacities of fresh‒cut red cabbage. The results showed that 50% extraction treatment remarkable maintained the quality of fresh‒cut red cabbage and enhanced the DPPH radical scavenging ability, the effect of Perilla frutescens extraction treatment was distinguished by suppressing the decrease of total phenolic, flavonoids, anthocyanin and Glutathione (GSH) contents and increasing the polyphenol oxidase (PPO), peroxidase (POD), phenylalanine ammonia lyase (PAL) activities. To sum up, 50% extraction treatment maintained physiological quality and improve the resistance of fresh‒cut red cabbage when stored at 4°C.
Keywords: Perilla frutescens, fresh‒cut, red cabbage, antioxidant enzyme activity
Perilla frutescens, belongs to Labiatae. P. frutescens family,1 is an important edible‒medicinal economic crops which widely distributed in Asia,2 such as China, Japan and South Korea. Perilla frutescens has been used as a medicinal plant for thousands of years in China for treating depression, anxiety, cough, allergy, poisoning, cancer and intestinal diseases3 because of its abundant phenolic compounds, flavonoids, rosmarinic acid, anthocyanin, vitamins and minerals contents.1,4
Red cabbage (purple‒leaved varieties of Brassica oleracea Capitates Group) is popular in the world because of its unique color and a great number of minerals, vitamins and antioxidant substances that are beneficial to human body.6,7 The unique color of red cabbage is formed by anthocyanins.8 Red cabbage is present mainly as fresh‒cut salads, fresh‒cut product was defined as fruits or vegetables that have been trimmed and/ or peeled, or cut into 100% usable product that is packaged to offer consumers high nutrition, convenience and flavor while still maintaining freshness. Fresh‒cut process involved peeling, cutting and other operations that may alter the integrity of tissue, disrupt physiological metabolism and cause stress,9 which will affect quality and shelf life of fresh‒cut products.
There are a large amount of antioxidant substance in Perilla frutescens and red cabbage, which are both beneficial to human. However, few studies have reported the effects of Perilla frutescens extraction treatment on fresh‒cut red cabbage. Therefore, the present study was conducted to investigate the effects of Perilla frutescens extraction application on fresh‒cut red cabbage at a temperature of 4°C.
Materials and sample preparation
Extraction procedure: Fresh leaves of Perilla frutescens were washed and dried, then filtered after homogenizing, and the liquid extraction was collected. Undamage and disease free red cabbage leaves (7‒10 outer layers) that were of a similar stage of color and maturity were cut into 0.5cm×5cm filaments, fresh‒cut red cabbage (1kg) were dipped into 50% Perilla frutescens extraction, 100% Perilla frutescens extraction and distilled water (as a control, CK) for 5min, respectively. The fresh‒cut red cabbage was drip dried then stored at plastic trays with sealed polyethylene film at 4°C.
Anthocyanin content: Anthocyanin content was determined according to Fan et al.10 with some modifications. The samples (1g) were homogenized with 5mL of precooked 1% HCl‒ethanol on ice then centrifuged at 12000g for 20min at 4°C, total anthocyanin content was measured using pH differential method, each treatment was conducted triple. Results were expressed as gram of cyanidin 3‒glucoside equivalents per kilogram of fresh weight.
Flavonoids content: Flavonoids content was determined according to Naheed et al.11 with some modifications. 0.3g of fresh‒cut red cabbage, 3.4mL of 30% ethanol, 0.15mL of 0.5mol L‒1 NaNO2 and 0.15mL of 0.3mol L‒1 AlCl3 were mixed. After 5min, 1mL of 1mol L‒1 NaOH was added to the reaction system. The absorbance was measured at 506nm. The flavonoid concentration was calculated from a calibration curve using rutin as the standard. All concentrations were expressed as grams per kilogram on a fresh weight basis.
Total phenolic content: Total phenolic content was determined by Folin‒Ciocalteu method.12 1g of sample was added into 5mL of precooked 1% hydrochloric acid‒methanol solution, then ice homogenized and centrifuged at 13000g at 4°C for 20min. The absorbance of supernatant at 760nm was measured and the level of total phenolics compounds was calculated using phenolics as a standard. Three replicates were conducted for each treatment.
Polyphenol oxidase (PPO) activity: For measurement of PPO activity, fresh‒cut red cabbage (5g) were homogenized with 20mL of ice‒cold citric acid buffer (0.2M, pH 6.8) then centrifuged at 10,000 g for 30min at 4°C. 2mL of citric acid buffer (pH 6.8), 1mL of 100mM 4‒methylcatechol and 2mL of the supernatant were used to carry out the assay. The increase in absorbance at 398nm at 25°C within 2min was recorded. The PPO activity was expressed as one unit. One unit of activity was defined as the amount of enzyme required to cause an increase of one absorbance unit at 398nm in one minute.13
Peroxidase (POD) activity: POD activity was determined by method described by Wang et al.14 2g (fresh weight) of tissue was ground in 6mL of 0.1M ice‒cold, sodium phosphate buffer (pH 7.8). The reaction solution contained 1mL of 0.1mol L‒1 sodium phosphate buffer (pH 7.8), 0.9mL of 0.2% Guaiacol, 0.5mL of enzyme extract, and 0.6mL of 0.3% H2O2. The change in absorbance at 470nm was recorded every 5s. The POD activity was expressed as one unit. One unit of activity was defined as the amount of enzyme required to cause an increase of one absorbance unit at 470nm in one minute.
Phenylalanine ammonia lyase (PAL) activity: For measurement of PAL activity, 0.4g of fresh‒cut red cabbage tissues was extracted with 6mL of extraction buffer (0.1mol L−1 borate, 0.1% mercaptoethanol; pH 8.8). The extracts were centrifuged at 10600g for 15min at 4°C. The assay was performed by 200μL of the extract added to 2.3mL of reaction buffer (borate 0.1M, 10mM phenylalanine; pH 8.8) at 40°C for 1 h and stopped by the addition of 0.5ml of 5M HCl. The change in absorbance was read at 290nm. The PAL activity was expressed as one unit. One unit of activity was defined as the amount of enzyme required to cause an increase of one absorbance unit at 290nm in one minute.15
Glutathione (GSH) activity: The GSH activity was measured according to Wang & Zhu.16 Samples (1g) were homogenized in 5mL 1%(w/v) saline buffer [1%(w/v) polyvinylpyrrolidone (PVP), 1mM ethylenediaminetetraacetic acid (EDTA)], then centrifuged at 11000g for 15min at 4°C, and the supernatants were collected for analysis. The method of GSH measurement is based on the generation of a yellow color when 5, 50 dithiobis (2‒nitrobenzoic acid) reacts with GSH. The absorbance was recorded at 412nm after 10min of reaction. GSH concentrations were expressed on fresh weight basis as mol/g.
DPPH radical scavenging ability: Fresh‒cut red cabbage was extracted with 50% ethanol, then centrifuged at 12000g for 20min at 4°C, and the supernatant was collected for determination. 0.1mL of supernatant was added to 2.9mL of methanol with 120mol L‒1 DPPH. An ethanolic solution of DPPH served as control. The result was calculated according to the following formula: DPPH radical scavenging ability (%)=100‒(absorbance of sample/absorbance of control)×100.17
Soluble protein: Soluble protein was determined by Coomassie brilliant blue method.1
Data analysis
All data were expressed as mean±standard error of the mean (SEM) and SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) was used to test for significant differences (P<0.05) in mean values between the control and extraction treatment.
Fresh‒cut market has rapidly expanded in recent decades due to its convenience and deliciousness. However, the appearance and texture of fresh‒cut products easily change during storage, such cut‒edge browning, chlorophyll and anthocyanin degradation.19 Many natural product extracts have been used to maintain the quality of fresh‒cut fruit and vegetable due to their safety, efficacy and great potential.20,21
Phenolic in plants are the main sources of antioxidant activity.22 The redox properties of phenolic compounds can act as reducing agents, hydrogen donors, singlet oxygen quenchers and metal chelators.23 Foods rich in phenolics have the function of retarding lipid oxidation degradation and controlling diseases such as heart disease through their strong oxidizing power.24 There was a loss in anthocyanin, flavonoids and total phenolic content in control and extraction treatments during storage, but 50% extraction treatment suppressed the decline of these substances compared with the control (P<0.05) (Figure 1‒3), which kept the nutrient content of fresh‒cut red cabbage. These results were accord with previous reports in fresh‒cut red cabbage treated by low doses Cu2+.25
PPO participates in the production of reactive oxygen species (ROS), quinones and hydroperoxides in cells and the aggregation of polyphenols, carbohydrates and proteins associated with plant defenses in cell walls.26 We found that extraction treatment stimulated the PPO activity (Figure 4), suggesting that extraction treatment may enhanced the resistance of plant. POD can eliminate excess H2O2.27 In our study, POD activity was induced by extraction treatment (Figure 5), higher POD activity can increase the antioxidant capacity of fresh‒cut red cabbage, Liang et al.28 have found that increased activities of POD and PPO can enhance the resistance in cassava.
The lignifications on cutting surface of red cabbage can resistant the infestation of pathogen, phenylpropanoids pathway regulate formation of callus, PAL plays a pivotal role in this pathway.29,30 In our research, PAL activity decreased in treatment group at the first day, then increased on 2d, following declined until 5d, then significantly increased, it is worth noting that the PAL activity in CK at 8d is lower than 0d, and the activity of PAL in extraction treatments were always higher than that of the control (P<0.05), and the effect of 50% extraction treatment was the best (Figure 6), indicating that the extraction treatment helps to enhance the resistance of the fresh‒cut red cabbage tissue, Our findings are in agreement with studies reported by Kumar & Lulai.30,31
GSH plays an important role in keeping cellular redox status,32 and has an significant function in antioxidant protection.33 We found that GSH content exhibits a fluctuating trend, a higher content were observed in red cabbage treated with extraction (Figure 7) (P<0.05). The DPPH free radical scavenging ability can reflect the antioxidant activity of fresh‒cut red cabbage. Compared with the control, the 50% extraction treatment significantly enhanced the ability of DPPH free radical scavenging at days 1 and 8 (Figure 8), these findings may related to higher contents of total phenolic, flavonoid, anthocyanin and GSH and higher level of POD activity in fresh‒cut red cabbage.
In our study, the soluble protein content of extraction treatment were significantly higher control (P<0.05), the effect of 50% extraction treatment was more pronounced (Figure 9). This result may be more related to the functional enzymes in extraction treated fresh‒cut red cabbage.
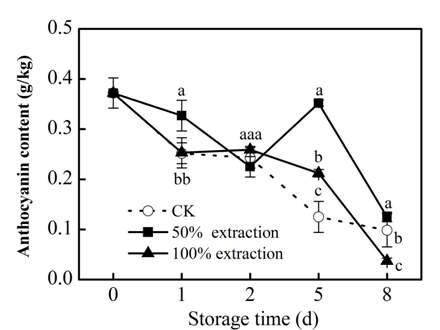
Figure 1 Effect of Extraction treatment on anthocyanin content of fresh‒cut red cabbage during storage. CK: control. Vertical bars are SD (n=3). The different letters (a, b, c) reveal significant difference at P<0.05 for each day..
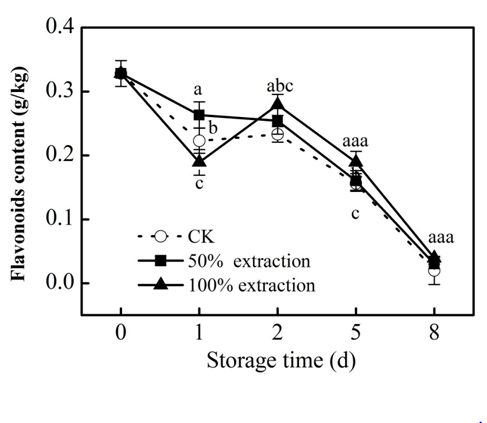
Figure 2 Effect of Extraction treatment on flavonoids content of fresh‒cut red cabbage during storage. CK: control. Vertical bars are SD (n=3). The different letters (a, b, c) reveal significant difference at P<0.05 for each day.
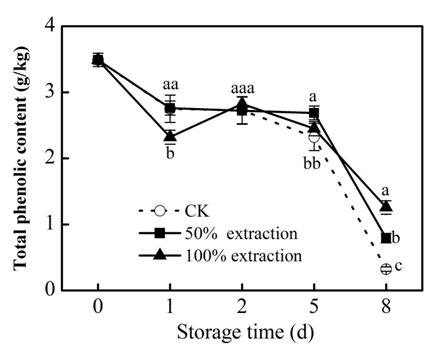
Figure 3 Effect of Extraction treatment on total phenolic content of fresh‒cut red cabbage during storage. CK: control. Vertical bars are SD (n=3). The different letters (a, b, c) reveal significant difference at P<0.05 for each day..
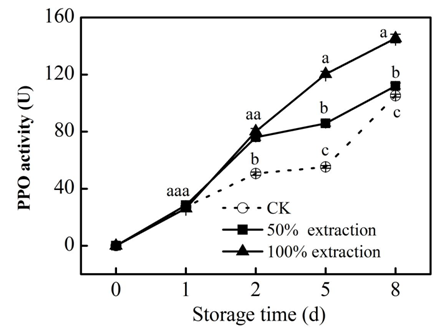
Figure 4 Effect of Extraction treatment on PPO activity of fresh‒cut red cabbage during storage. CK: control. Vertical bars are SD (n=3). The different letters (a, b, c) reveal significant difference at P<0.05 for each day.
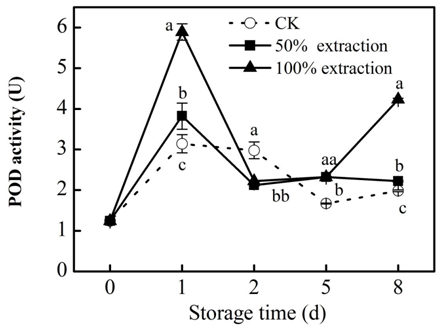
Figure 5Effect of Extraction treatment on POD activity of fresh‒cut red cabbage during storage. CK: control. Vertical bars are SD (n=3). The different letters (a, b, c) reveal significant difference at P<0.05 for each day.
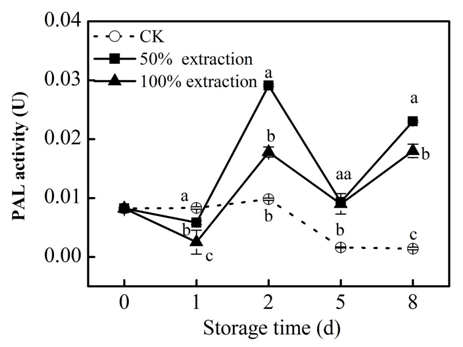
Figure 6 Effect of Extraction treatment on PAL activity of fresh‒cut red cabbage during storage. CK: control. Vertical bars are SD (n=3). The different letters (a, b, c) reveal significant difference at P < 0.05 for each day.
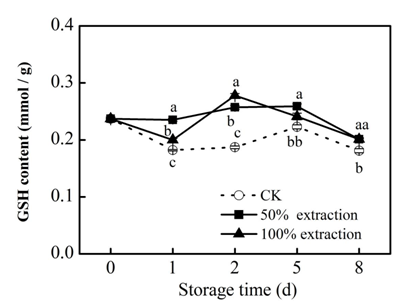
Figure 7 Effect of Extraction treatment on GSH content of fresh‒cut red cabbage during storage. CK: control. Vertical bars are SD (n=3). The different letters (a, b, c) reveal significant difference at P<0.05 for each day
In conclusion, we found that Perilla frutescens extraction treatment maintained the quality of fresh‒cut red cabbage, the effect of 50% extraction treatment is better than 100% extraction treatment, the mechanism of these were the likely to hold the content of antioxidant components, enhance the activity of antioxidant enzymes, and improved fresh‒cut red cabbage tissue resistance.
This study was supported by the Thirteenth Five-Year Plan’ for National Key R&D Program of China (Grant No. 2016YFD0400903).
The author declares that there is none of the conflicts.

©2018 Jiang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.