MOJ
eISSN: 2381-182X


Research Article Volume 5 Issue 3
1National Centre of Excellence in Analytical Chemistry, University of Sindh, Pakistan
2HEJ Research Institute of Chemistry, International Centre for Chemical and Biological Sciences, Pakistan
Correspondence: Farah Naz Talpur, National Centre of Excellence in Analytical Chemistry, University of Sindh, Jamshoro76080, Pakistan, Tel +92-222-772065, Fax +92-22-9213431
Received: October 30, 2016 | Published: December 5, 2017
Citation: Talpur MK, Talpur FN, Balouch A, et al. Analysis and characterization of anthocyanin from phalsa (grewia asiatica). MOJ Food Process Technol. 2017;5(3):299-305. DOI: 10.15406/mojfpt.2017.05.00127
Anthocyanins (ACNs) are water-soluble plant pigments having a vital role in plant physiology and human health. Grewia asiatica L., genus (Tiliaceae) is a fruit producing shrub, originating from south Asia (Pakistan, India), East (Cambodia), cultivated mainly for its comestible fruit and well reported diverse medicinal uses. The current study was the first attempt to characterize ACNs from G. asiatica, native to Pakistan. Results revealed that maximum anthocyanin extraction yield (1193.8µg/g) was obtained with acidified methanol as compared to other investigated solvents (water, methanol, ethanol and their binary mixture). Total seven ACNs were identified including non-acylated (delphinidin-3-O-glucoside, peonidin-3-O-glucoside, pelargonidin-3-O-malonyl glucoside), acylated (cyanidin-peonidin-and pelargonidin-3-O-6”-acetylglucoside) and pyranoanthocyanin (Malvidin-3-O-glucoside pyruvic acid). Among them cyanidin-3-O-(6"acetylglucoside) was the major ACN comprising 44-63% (695µg/g) of total ACNs composition followed by peonidin-3-O-glucoside consisting of 3-30% (163.6µg/g) and pelargonidin-3-O-(6”-acetyl glucoside) 8-14% (140.4µg/g). Moreover, the study of ACNs stability at the temperature 10-55°C, testified the better temperature tolerance by ACNs under dark at temperature ranging, 10-40°C. Hence, the results revealed that G. asiatica is a potent fruit rich in ACNs and can be used as food colorant and nutraceutical.
Keywords:grewia asiatica, cyanidin-3-o-6”acetyl glucoside, pH differential method, LC-MS/MS
ACN: Anthocyanins; Cy: Cyanidin; Dp: Delphinidin; Pt: Petunidin; Pn: Peonidin; Pg: Pelargonidin; Mv: Malvidin
Anthocyanins (Greek anthos, “flower” and Greek kyanose, “blue”), an extensive group of flavonoids, are water-soluble plant pigments.1 They exist in vacuoles of plant cells and are responsible for vigorous blue, purple, and red colours of various botanic organs such as flowers, grains, and fruits.2
They occur primarily as glycosides or acylglycosides of their respective aglycone anthocyanidins. There are almost seventeen natural anthocyanidins; however, only 6 of them, cyanidin, delphinidin, petunidin, peonidin, pelargonidin, and malvidin are universally distributed.3 Over 600 naturally occurring ACNs have been reported differing in (a) the number and position of hydroxyl and methoxyl groups in the basic anthocyanidin skeleton, (b) identity, number and positions at which sugars are attached and (c) the extent of sugar acylation and identity of acylating agent.3 They differ from other natural flavonoids on the basis of colour ranges that can be derived from them and their4 capability to form resonance structures through pH variation.5 Therefore, ACNs are regarded as potential candidates for natural colorants in the food industry.6,7 Similarly, in the past years, questions were raised regarding the safety of synthetic colorants due to their toxicity, thus the interest in natural pigments has been increased significantly as a consequence of both the legislative action and consumer awareness.8
ACNs are considered as substantial dietary compounds also, owing to their potent free radical scavenging and antioxidant nature in vitro leading to their antihypertensive and anticarcinogenic4 behaviour against oral, esophageal and colon cancer.5 In addition, ACNs could promote sharp-sightedness, immune response, blood circulatory system and protection against cardiac diseases.6 The research has shown that acylated ACNs may behave quite differently from their nonacylated counterparts, in terms of their stability and bioavailability; (Stoner et al., 2005), hence it is not reasonable to consider all ACNs equally. Therefore, knowledge regarding the concentrations and daily intake of specific ACNs is essential.
The main sources of ACNs are fruits and some vegetables such as grapes, blackberries, blueberries, egg-plants (aubergine) and avocado.9 Recently, indigenous fruits such as Syzyguim cumini have been found as potential candidates for polyphenols and flavonoids, particularly. In this context Grewia asiatica L., (called Phalsa locally)10,11 which is agronomically crucial indigenous plant, native to South Asia (Pakistan and India) and Cambodia, cultivated mainly for its comestible fruit and well-reputed diverse medicinal uses.12,13 Also, it is used to manufacture soft drinks, jams, pies, squashes and chutneys. Incontrovertible evidence has been documented that G. asiatica fruit has an antioxidative and antiradical capacity,14 particularly its peel, mush and seed. In this regard, assessed the antioxidant activity of G. asiatica leaves extract while Rymbai, H et al.8 evaluated its greater antiradical potential up to 95.87% in Swiss albino mice.8 Later on, they found a significant hepatoprotective efficacy of its fruit extract in the same organism.8 In addition declared G. asiatica fruit extract as a rich source of ACNs (by evaluating total ACNs) and found its effective antimicrobial activity against four different species of gram-positive and negative bacteria. Despite its diverse use, it has suffered remarkable disregard, as is evident from the lack of literature about the described plant.11 Therefore, it was necessary to strengthen the research about the G. asiatica fruit pigment in order to promote the industrialization of its fruit products.
Keeping in mind the therapeutic importance and possible industrial utilization as a natural colorant, the present study is focused on the identification and quantification of individual ACNs from G. asiatica fruit. Also, their solubility in different solvents and stability at various temperatures under the influence of light and dark have been highlighted.
Standards and solvents
Analytical grade methanol and ethanol (for extraction purpose) were procured from Sigma Aldrich (Darmstadt, Germany), while for HPLC analysis and cleanup procedure, HPLC grade chemicals such as Methanol, formic acid, ethyl acetate and hydrochloric acid (HCl) were purchased from Fischer scientific Ltd (Lough borough, UK). Standards of the 3-O-α-glucoside chloride of pelargonidin, cyanidin, peonidin, delphinidin, petunidin and malvidin and cyanidin-3-O-rutinoside (Keracyanin Chloride, 99% pure) were obtained from Applicam (Darmstadt, Germany).
Samples collection and storage
Ripe fruit samples of G. asiatica (dark purplish and hemispherical single-seeded) were purchased from local markets of Hyderabad, Sindh province, Pakistan; placed into the cold insulated sampling box immediately followed by whole fruit homogenization and storage at -20°C. The homogenized samples were lyophilized for 48 h using Trio science lyophilize Model TR-FD-BT-50 (Tokyo, Japan) and stored at -4°C until analysis.
Anthocyanin extraction and purification
ACNs were extracted from G. asiatica fruit samples following a reported method with slight modifications. Briefly, 10 ml acidified methanol (HCl, 0.1% v/v) was added to 1g of lyophilized sample and incubated (30 rpm) at 20°C for 24 h in dark followed by separation of supernatant through centrifugation (5000 rpm) at 4°C for 20 min.
For purification of filtered supernatant, a standard procedure15 was employed. In brief, ACN extract was loaded on C18 Sep-Pak cartridge (Waters Associates, Milford, USA), preconditioned with deionized water and methanol (HPLC grade), and washed with 5-fold volume of ultra-pure acidified water (HCl, 0.01% v/v) and ethyl acetate followed by elution with methanol containing 0.1% HCl. The ACN solution was collected and condensed at 40°C using a rotary evaporator under vacuum for the spectroscopic and chromatographic analysis. All analyses were performed in triplicate.
Solvent study
In order to obtain the ACNs extract with maximum concentration, different acidified (HCl, 0.1% v/v, for equal visual color strength) extraction solvents like water, methanol, ethanol and binary mixture (1:1) of acidified methanol with water and ethanol were investigated.
Determination and quantification of total anthocyanin contents
Total ACN contents in G. asiatica samples were determined by UV-Visible Spectrophotometer (Biochrom Libra S22) following previously reported a simple and cost effective AOAC pH differential method.16 It is well-known that ACNs undergo reversible structural transformations with pH alteration, manifested by amazingly different absorbance spectra. At pH 1.0 the coloured oxonium form while at pH 4.5 colourless hemiketal form is prevailed. The pH-differential method, is based on that principle8 and permits accurate and rapid measurement of total ACNs content, even in the presence of other interfering agents.
To estimate the concentration of monomeric ACNs, the absorbance of methanolic crude extracts, diluted in potassium chloride (0.025 M, pH 1.0) and sodium acetate buffer (0.4 M, pH 4.5), was measured in curette of 1 cm path length at 520 and 700 nm and ambient temperature (23°C) against a blank containing acidified methanol and aforementioned buffers. The resultant absorbance (A) was calculated as follows:
The monomeric ACN concentrations were calculated, according to the following formula
Where the molecular weight (MW = 630.98) and molar absorptive (ε = 26,900) of cyanidin-3-O-rutinoside, and dilution factors (DF) of concerned samples were used.
Stability study of anthocyanin
Temperature and light are among the most important factors that influence the ACNs stability and deterioration.8 The thermal stability was investigated in the range of 10-55°C in dark and light for 144 h (6 days) using temperature control New Brunswick Innova 4200 Incubator-Shaker (Gaithersburg, Maryland) equipped with incandescence light bulb, In typical procedure,8 four screw-topped glass vials containing samples of ACNs extract enclosed with aluminium (to protect from light) and sealed with para film, were placed in the dark at temperatures mentioned above. Meanwhile, four other samples without covering of aluminium foil were exposed to light at same temperature range. The stability was studied by recording UV-visible spectral variation at 520 and 700 nm.
HPLC-DAD analysis and quantification of individual anthocyanins
Chromatographic analyses were performed following a reported method,17 using auto sampler UV 000LP series HPLC (Thermo electronic corporation) equipped with diode array detector. Ten micro liter sample was injected and separated with Hypersaline gold (Thermo scientific) analytical C18 column (250 × 4.6 mm, 5 µm) using a mixture of methanol and formic acid solution (5% v/v) as eluent at the ratio of 15 : 85 with flow rate of 1 ml min-1. . The detection wavelengths were set to 520, 530 and 540 nm.
LC-MS/MS confirmation of individual anthocyanins
The confirmation and identification of individual ACNs were established with Agilent (model 6460) triple quadruple liquid chromatography/mass spectrometer (LC-MS/MS). The analysis was made in ESI interface with nebulizer gas pressure 45 psi with flow rate of 12 ml min-1. . The capillary voltage was kept at 3500 V and the tandem mass spectrometry scan was accomplished in the range of 350 to 1500 m/z.
Statistical Analysis
Analysis of variance (ANOVA) was used to analyze data by Tukey’s post-test, data was considered significant at P < 0.05 using SPSS version 18.0 (SPSS, Inc., 2009, Chicago, IL, USA). The association among variables was evaluated by Pearson’s correlation.
Solvent effect on anthocyanin extraction
Solvent extraction is considered as the most employed method to prepare fruit samples. In this connection, we tried acidified (HCl, 1% v/v) water, methanol, ethanol and binary mixture (1:1) of acidified methanol with water and ethanol.
The best extraction efficiency was demonstrated by methanol with 1193.8 µg/g extract (P < 0.05) as it is evident from the bar graph. Binary solvents i.e. 50% v/v acidified methanol and ethanol also showed satisfactory performance Figure 1 and no remarkable difference (P > 0.05) was observed between the extraction capability of binary and single solvent systems.
The quantification of anthocyanin in G.asiatica fruit was done by plotting the standard calibration graph of Keracyanin chloride (Cyanidin-3-O-Rutinoside). In Figure 2 the peak at similar wavelength as shown by Cyanidin-3-O-Rutinoside confirmed that anthocyanins from G.asiatica fruit pigment contain the same structural unit as cyanidin.
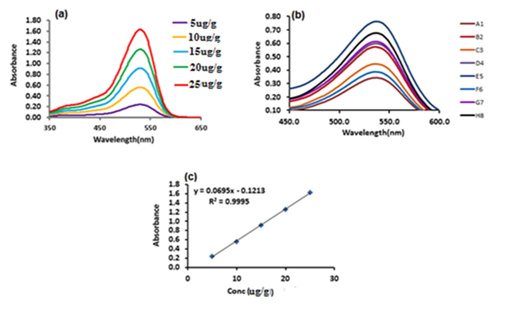
Figure 2 UV-Visible spectra of (a) Keracyanin chloride (Cyanidin-3-O-Rutinoside) from 5 to 25µg/g (b) Anthocyanin extract of different G.asiatica samples (c) Linear calibration graph of Keracyanin chloride (cyaniding-3-O-rutinoside) standard.
Thermal Stability of anthocyanins at different storage conditions
Total eight samples of ACNs extracts were subjected to temperature ranging 10–55°C, the results exhibit that the degradation of ACNs, exposed to light, was 28.55% at temperature between 10 to 25°C and 60% between 25 to 55°C (P ˂ 0.05) as it can be seen in (Figure 3(A)). ACNs, placed in dark, also showed almost the same stability at temperature 10 to 25°C (P ˃ 0.05), however, a noticeable reduction in stability occurred with 50% degradation by increasing temperature from 25 to 55°C as it is obvious from Figure 3(B). It has been reported by other authors also temperature elevation leads to the acceleration of ACNs destruction.5

Figure 3 Stability of G.asiatica anthocyanin extract in (a) presence of light and (b) dark with variable temperature and time.
In addition, ACNs in dark were found to be comparatively more thermally stable (for 96 h) at 40°C which is in agreement with the degradation behavior of ACN extracts of black grape skins at identical temperature.18 Thus light was found to be deleterious to ACNs. Horbowicz and his colleagues also have documented a short half-life (197 days) for grape ACNs in light and long half-life (416 days) in dark at 20°C.
Peak Identification and Quantitation
The comparison of Analytes retention times and mass spectral data with those of standards and literature revealed that in the present study, for the first time seven ACNs of G. asiatica fruits have been identified and quantified (Figure 4).
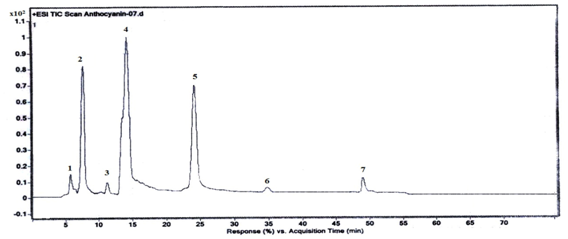
Figure 4 Total ion chromatogram of G.asiatica freeze dried extract showing anthocyanins separation (1) Delphinidin 3-O- glucoside (2) Peonidin 3-O- glucoside (3) Peonidin-3-O (6"-acetyl glucoside) (4)Cyanidin-3-O(6"acetyl glucoside) (5) Pelarigonidin-3-O-6”acetyl glucoside (6) Pelarigonidin-3-O-malonyl glucoside) (7) Malvidin-3-O-glucoside pyruvic Acid.
The tabulated LC-MS/MS data of ACNs composition in eight different samples of G. asiatica extracts show three categories of ACNs, on substitution basis as non-acylated anthocyanin, acylated anthocyanin and pyranoanthocyanin Table 1. Cyanidin-3-O-(6"acetyl glucoside) was the major ACN comprising 44-63% (695 µg/g) of total ACNs composition followed by 3-30% (163.6 µg/g) peonidin-3-O-glucoside and pelargonidin-3-O-(6”-acetyl glucoside) 8-14% (140.4 µg/g). Small quantities of delphinidin-3-O-glucoside, pelargonidin-3-O-malonyl glucoside, peonidin-3-O-(6"-acetyl glucoside) and malvidin-3-O-glucoside pyruvic acid were also detected as shown in Table 1. Xianli et al.3 andFan‐Chiang et al.14 have reported that Cyanidin-3-O-(6"acetyl glucoside) was the main ACNs found in Black raspberry (669 mg/ 100 g) and black barriers (137 mg/100 g). In addition has also reported the presence of cyanidin 3-glucoside, cyanidin 3-rutinoside, pelargonidin 3-glucoside and pelargonidin 3-rutinoside and pelargonidin 3-acetylglucoside in strawberry samples collected from Salamanca, Spain.
|
Anthocyanin (µg/g) |
GA1 |
GA2 |
GA3 |
GA4 |
GA5 |
GA6 |
GA7 |
GA8 |
|
Delphinidin 3-O-glucoside |
28.6 ±1.1a |
37.2±1.3b |
36.3±1.5b |
55.5±2.1c |
50.5±2.5c |
13.1±0.6d |
85.7±4.1e |
34.5±1.6b |
|
Peonidin 3-O- glucoside |
132.7±6.6a |
80.5±4.0a |
60.7±3.0c |
63.7±3.1c |
37.2±1.8d |
116±5.7e |
334.2±16.6f |
320.6±15.8f |
|
Pelarigonidin-3-O-malonyl glucoside |
45.0±2.24a |
62.4±3.1b |
27.9±1.3c |
76.3±3.8d |
85.6±4.2e |
36.5±1.8f |
17.6±0.8g |
19.9±0.9g |
|
Cyanidin-3-O(6"acetyl glucoside) |
605±30.0a |
584.1±29.1b |
636±31.2a |
671.4±33.21c |
760.8±38.0d |
636.9±31.8a |
470.5±23.2e |
504.4±25.1f |
|
Peonidin-3-O (6"-acetyl glucoside) |
51.6±2.5a |
107.5±5.3b |
86±4.1c |
69.1±3.4d |
73.7±3.6e |
44.7±2.2a |
33.9±1.7e |
34.5±1.7e |
|
Pelarigonidin-3-O-(6”acetyl glucoside) |
78±3.8a |
160.6±8.02b |
118.3±5.9c |
126.2±6.2d |
126.4±6.31d |
126.497±4.8f |
118.2±5.90c |
158.6±7.9b |
|
Malvidin-3-O-glucoside pyruvic acid |
51±2.5a |
72.9±3.6b |
76.5±3.8b |
52.0±2.4a |
59.7±2.9a |
69.5±3.4b |
61.7±3.1c |
79.1±3.9b |
Table 1 Anthocyanin composition in different samples of Grewia asiatica
Data is presented as Mean±standard deviation; different superscript in a column shows significant differences at P < 0.05
Here it is worth mentioning that some authors have proposed that ACNs are therapeutically more efficient in mixture form. The data of LC/PDA/ESI-MS and anthocyanin composition in eight different samples of G.asiatica extracts is summarized in Table 1.
Fragmentation patterns of G.asiaticaanthocyanins
Molecular weights and fragmentation behaviour of ACNs were determined by LC-MS/MS as previously reported18 and it was found that amongst the seven identified ACNs, three were nonacylated (peak 1, 2 and 5), other three were acylated (peak 3, 4 and 6) and one was Pyranoanthocyanin (peak 7) as depicted in Table 2.
Peak No: |
Retention Time (min) |
[M]+ ( m/z) |
MS/MS ( m/z) |
Anthocyanin |
1 |
5.716 |
465 |
303 (Dp) |
Delphinidin3-O- glucoside |
2 |
7.495 |
463 |
301 (Pn) |
Peonidin 3-O- glucoside |
3 |
11.143 |
505 |
301 (Pn) |
Peonidin-3-O (6"-acetyl glucoside) |
4 |
14.104 |
491 |
287 (Cy) |
Cyanidin-3-O(6"acetyl glucoside) |
5 |
24.161 |
519 |
433/271 (Pg) |
Pelarigonidin-3-O-malonyl glucoside |
6 |
34.814 |
475 |
271 (Pg) |
Pelarigonidin-3-O-(6”acetyl glucoside) |
7 |
49.116 |
561 |
493/331 (Mv) |
Malvidin-3-O-glucoside pyruvic Acid |
Table 2 Mass spectral data of anthocyanins in phalsa (Grewia asiatica)
Peak 1 displayed molecular ion peak [M]+ of delphinidin-3-O-glucoside at 465 m/z and fragment ion peak [M - 162]+ at 303 m/z due to the removal of glucoside unit. Peak 2 had molecular cation of peonidin-3-O-glucoside at 463 m/z and fragment ion [M - 162]+ at 301 m/z for peonidin anthocyanidin backbone. Similarly, peak-5 showed molecular cation of pelarigonidin-3-O-malonyl glucoside at 519 m/z along with fragment ions at m/z 433 [M – 186]+ and 271 m/z due to the cleavage of malonyl and glucoside groups respectively (Figure 5).
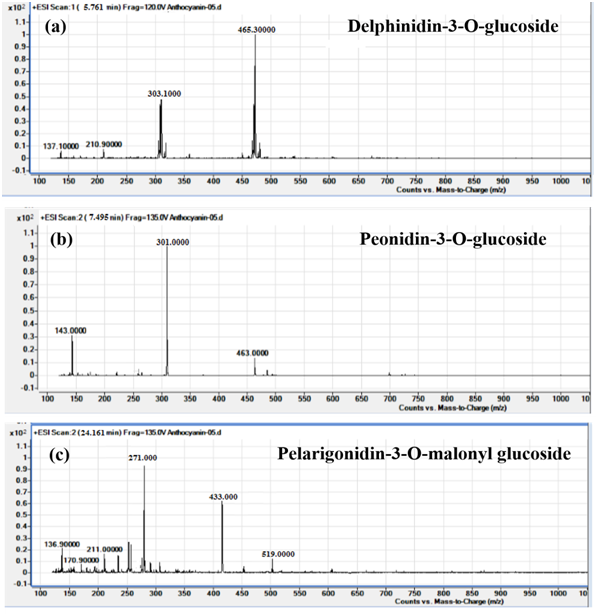
Figure 5 Mass fragmentation of non-acylated anthocyanins, (a) delphinidin-3-O-glucoside, (b) peonidin-3-O-glucoside and (c) pelarigonidin-3-O-malonyl glucoside.
Acylation of anthocyanin sugar moieties brings about an increase in molecular weight and decrease in polarity. Therefore, in peak -3 the molecular ion of peonidin-3-O-(6”-acetyl glucoside) appeared at 505 m/z, higher than its nonacylated counterpart, with fragment ion [M+204] of acetyl glucoside at 301 m/z. Peak -4 yielded M+ of cyanidin-3-O-(6”-acetyl glucoside) at 491 m/z and fragment ion of acetyl glucoside at 287 m/z. Another acylated anthocyanin,19,20 pelargonidin-3-O-(6”-acetyl glucoside) was specified by peak-6 with molecular ion peak [M]+ at 475 m/z and fragment ion [M-204]+ at 271 m/z (Figure 6).
Peak 7 demonstrated [M]+ of malvidin-3-O-glucoside pyruvic acid at (Figure 7) 561 m/z and two main fragments at 493 and 331 m/z (due to the removal of malvidin) with the difference of 162 due to the loss of glucoside group.
The present study reveals that Phalsa (Grewia asiatica) is a promising indigenous fruit in terms of anthocyanins content. The results showed that cyanidin-3-O(6"acetyl glucoside) was the abundant anthocyanins comprising 44-63% of total anthocyanin followed by peonidin 3-O- glucoside (3-30%) and pelarigonidin-3-O-(6”acetyl glucoside) (8-14%). Other anthocyanins identified were delphinidin 3-O- glucoside, pelarigonidin-3-O-malonyl glucoside, peonidin-3-O (6"-acetyl glucoside) and malvidin-3-O-glucoside pyruvic acid contributing 16-25% of total anthocyanin. Acidic methanol was found as a best solvent for extraction of anthocyanins from Grewia asiatica. In addition extracted anthocyanins were more stable in dark than exposed to ultra violet light. In dark extract was stable at temperature range of 10-40 ̊C. Overall current study indicates that Phalsa (Grewia asiatica) is a promising indigenous fruit and has potential to be explored as food colorant and nutraceuticals using food grade solvent extraction strategies.
Marvi Kanwal Talpur greatly acknowledges National Center of Excellence in Analytical Chemistry, University of Sindh Jamshoro for providing her monthly stipend for this research work. No other funding resources were available during the completion of this work.
All authors declare that they have no conflict of interest.

©2017 Talpur, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.