MOJ
eISSN: 2381-182X


Research Article Volume 3 Issue 2
1Department of Chemistry, University of Warsaw, Poland
2Institute of Chemistry and Technical Electrochemistry, Poznan University of Technology, Poland
Correspondence: Krystyna Pyrzynska, Department of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland
Received: October 28, 2016 | Published: December 5, 2016
Citation: Sentkowska A, Jeszka-Skowron M, Pyrzynska K. Comparative studies on the antioxidant properties of different Green coffee extracts. MOJ Food Process Technol. 2016;3(2):296-302. DOI: 10.15406/mojfpt.2016.03.00071
Green coffee infusions has begun to enjoy a great popularity due to a pro-health status of antioxidants rich products. Different assays - determination of reducing power (Folin-Ciocalteau, CUPRAC methods), DPPH radical scavenging activity, metal chelating activity - were compared to get a better estimate of the antioxidant properties of green coffee infusions from robusta and arabica types of different geographical origin. The content of some phenolic acids were also determined by HPLC-MS. The content of 5-O-caffeoylquinic acid, the main component in robusta type varied from 80.0 to 172.7mg/L, while in arabica coffees this range was narrower (133.0-176.1mg/L). Ferulic acid was found at very similar level (12.5-12.8mg/L) only in robusta coffees. In order to correlate the obtained results using different assays the antioxidant index was calculated. Among studied extracts, robusta green coffee from Laos, with the highest content of chlorogenic acid, had the most potent antioxidant capacity.
Keywords: green coffee, antioxidant properties, reducing power, radical scavenging activity, antioxidant index
In the last decade, determination of antioxidant activity and the total content of antioxidants in foods, beverages, herbal extracts and dietary supplements has been in wide demand.1,2 This activity is related with compounds capable of protecting a biological system against the potentially harmful effect of processes or reactions than cause excessive oxidation, involving reactive oxygen species.3 In addition, these compounds can exert antioxidant effects acting as reducing agents and metal chelators.
These are the two primary types of coffee cultivated for drinking. Coffe arabica (commonly known as Arabica coffee), which accounts for about 75% of world production, with the remaining ~25% coming from Coffee canephora (commonly known as Robusta coffee).4 Arabica usually comes from South America (mostly from Brazil) as well as from upland and mountain areas of East Africa, while Robusta from South Asia (mainly from Vietnam) and lowland of Central and West Africa. Green coffee beans contain a variety of compounds, including a large amount of chlorogenic acids (CGA), which are the esters of caffeic,ferulic or p-coumaric acids with quinic acid. The main CGA, 5-O-caffeoylquinic acid (5-O-CQA) and its isomers 3-O-CQA and 4-O-CQA constitute almost 83% of total CGA content in green coffee.5 The content of these compounds is dependent on the species, the variety of coffee and the processing conditions.6-9 During roasting, the green beans are heated at 200-240 oC for 10-15 min depending on the degree of roasting required. It leads to considerable changes in the chemical composition; the content of some compounds such as amino acids, reducing sugars, chlorogenic acid and water decreasing, while also new compounds are formed due to the Maillard reaction, such as melanoidins.7
There is a great interest of quantification of antioxidants and determination of antioxidant capacities of a number of specific food compounds. The antioxidant capacity of natural products is considered as a significant parameter determining their dietary value. Many methods have been developed for these purposes.10-12 They differ from each other in terms of reaction mechanisms, oxidant and target/probe species, reaction conditions and expression of results. Currently, there is no single antioxidant assay for food labeling because of the lack of standard quantification methods.2 Thus, to properly measure antioxidant capacity of a given sample, the multidirectional analysis should be done using several methods.
The purpose of this study was to evaluate and compare the antioxidant activity of several green coffee brews of different geographical origin. Different assays - determination of reducing power (Folin-Ciocalteau and CUPRAC methods), DPPH radical scavenging activity and metal chelating activity - were used for this purpose. The contents of chlorogenic, caffeic and ferullic acids were determined by HPLC-ESI-MS.
Reagents and apparatus
The commercial standards of phenolic acids, Folin-Ciocalteu`s reagent and DPPH (2,2-diphenyl-1-picryhydrazyl radicals) as well as the other chemicals were purchased from Sigma (Steinheim, Germany). Methanol and acetonitrile were of HPLC gradient grade from Merck (Darmstadt, Germany). Ultrapure water from Milli-Q system (Millipore, Bedford, MA, USA) with a conductivity of 18 MQ was used in all experiments. All solutions were filtered through 0.45 μm membranes (Millipore) and degassed prior to use.
Spectrophotometric determination were performed on a Perkin Elmer model Lambda 20 UV-VIS spectrophotometer with cuvettes of 1 cm length. Spectra were recorded in the range from 220 to 600 nm with 0.2 nm resolution. Data were processed with WinLab software.
Chromatographic analysis was performed with a Shimadzu LC system consisted of binary pumps LC20-AD, degasser DGU-20A5, column oven CTO-20AC, autosampler SIL-20AC, detector UV SPD 20A connected to 3200 QTRAP Mass spectrometer (Applied Biosystem/MDS SCIEX). A MS system equipped with electrospray ionization source (ESI) operated in negative-ion mode and a quadrupole mass analyser in a scan mode from 50 to 1500 m/z. ESI conditions were following: capillary temperature 450 °C, curtain gas at 0.3 MPa, auxiliary gas at 0.3 MPa, negative ionisation mode source voltage - 4.5 kV. Nitrogen was used as curtain and auxiliary gas
For chromatographic analysis KinetexTM (Phenomenex) C-18 column (100 x 2.1 mm, 2,6 µm) at 30 °C was used and gradient elution with 2 mM formic acid (pH 2.8) as eluent A and acetonitrile as eluent B were. The mobile phase was delivered at 0.2 mL/min in linear gradient mode: 0-5 min. 20% B, 10 – 15 min 25 % B, 20-25 min 30% B, 30-31 min 90% B, 32 min 20% B.13 Compounds were identified by comparing retention time and m/z values obtained by MS and MS2 with the mass spectra from standards tested under the same conditions.14 Quantification of compounds was done from the calibration curves obtained in selected reaction monitoring (SRM) mode.
Samples and coffee extracts preparation
Twelve green coffee beans of different geographical origin: Coffea arabica (Brazil, Ruanda, China, Laos) and Coffea robusta (Vietnam, Vietnam decaffeinated and Vietnam steamed, India, Indonesia, Laos, Uganda Sc and Uganda Bugishu) were obtained from Strauss Café, Poland. 0.5 g of milled beans were extracted by 20 mL of distilled water (at 94 °C) for 10 minutes. Then the solution was cooled to room temperature evaporated (5 minutes, 4500 rpm) and decanted. The extracts were lyophilized (Lyophilizator Alpha 1-2 LD plus; Martin Christ, Germany). Before analysis 50 mg of the extracts were dissolved in 1 mL of Millipore water and filtered through 0.2 µm polytetrafluoroethylene syringe filter from Agilent Technologies (Santa Clara, CA, USA).
Antioxidant properties of coffee brews
Reducing power of green coffee brews were determined by the Folin-Ciocalteau assay and CUPRAC (cupric ion reducing antioxidant capacity) method. The Folin-Ciocalteu assay was conducted according to Chua et al.15 with a slight modification. Briefly, 1 mL of coffee extract was mixed with 0.1 mL of Folin-Ciocalteu’s reagent and 0.9 mL of water. The solution was allowed to stand for 5 min, and then 1 mL of sodium carbonate (7%, w/v) and 0.4 mL of water were added and 30 more min was allowed for stabilization of the blue color formed. The absorbance against a reagent blank was measured at 765 nm. The data were expressed as gallic acid equivalent (GAE) or Trolox equivalent (TRE).
For CUPRAC method, the assay described by Apak et al. [16] was adopted. To 1 mL of CuCl2 solution (1.0·10-2 M) 1 mL of neocuproine alcoholic solution (7,5·10-3 M) and 1 mL of 1 M NH4AC buffer (pH 7) were added, followed by 0.5 mL of coffee infusion and 0.6 mL of water. The mixture was incubated in a water bath at a temperature of 50 oC for 20 min, followed by cooling under running water. Absorbance against the reagent blank was measured at 450 nm. The calibration curve was built up with trolox and the antioxidant activity was expressed as trolox equivalent (TRE).
DPPH radical scavenging activity
DPPH radical scavenging activity of the coffee infusions were determined according to the method of Pękal & Pyrzynska.17 Stock solution of DPPH• (3·10-5 M) were prepared in methanol with acetate buffer (0.1 M, pH 5.5) at 60:40 volume ratio. 2.4 mL DPPH solution was mixed with 0.1 mL of a given infusion and immediately the change of absorbance at 539 nm was recorded over time against the blank. The results were expressed as the percentage of inhibition of the DPPH• according to expression: (A0-At)/Ao x 100, where Ao is the initial absorbance and At is the absorbance at increasing time t. The DPPH scavenging activity was also reported as Trolox equivalent (TRE).
Metal chelating activity
The ferrous-ion-chelating (FIC) assay was adapted from Tang et al.18 FIC ability was determined by mixing FeCl2 (2 mM, 0.1 mL) with different dilutions of extracts (1 mL), followed by ferrozine (5 mM, 0.2 mL). Absorbance was measured at 562 nm after 20 min. The ability of extracts to chelate ferrous ions was calculated as chelating effect % = (1-Asample/Acontrol) x 100.
Statistical analysis
The extracts for experiments were prepared separately from three portion of a given coffee. Each value is the mean of triplicate determinations. Statistical analysis was conducted using the software package STATISTICA 8.0 for windows from Statsoft. A P-value of less than 0.05 was considered statistically different.
HPLC-MS analysis of coffee extracts
Three phenolic compounds were identified and quantified in the extracts of studied green coffees, with 5-O-caffeoylquinic acid (5-O-CQA) as the main component (Table 1). The content of 5-O-CQA in robusta type varied from 80.0 to 172.7 mg/L, while in arabica coffees this range was narrower (133.0 - 176.1 mg·L-1). Chlorogenic acids of coffee infusion are responsible for flavour and astringency. During roasting processes, a part of these compounds is transformed into quinolactones and degraded into low molecular weight compounds or even reduced depending of roasting parameters and coffee preparation.4,5
Coffee |
5-O-CQA |
Caffeic acid |
Ferulic acid |
Robusta |
|||
Vietnam |
113.1±3.39a |
0.95±0.029a |
12.56±0.44a |
Vietnam decaffeinated |
115.3±3.44a |
1.14±0.034b |
< LOD |
Vietnam steamed |
80.0±2.39b |
<LOD |
12.61±0.38a |
India Cherry |
135.7±4.06c |
<LOD |
12.60±0.35a |
Indonesia |
128.1±3.82d |
5.21±0.156c |
12.76±0.37a |
Laos |
172.7±5.14e |
0.46±0.014d |
12.64±0.32a |
Uganda |
157.1±4.67f |
4.23±0.126e |
12.46±0.29a |
Uganda Bugishu |
160.4±4.80f |
<LOD |
12.55±0.30a |
Arabica |
|||
Brasil |
161.6±4.78f |
<LOD |
<LOD |
Laos |
133.0±3.99c |
<LOD |
<LOD |
China |
145.3±4.31g |
<LOD |
<LOD |
Ruanda |
176.1±5.24e |
<LOD |
<LOD |
Table 1 Concentration (in mg·L-1) of selected phenolic acids in studied green coffee infusions
< LOD, below the limit of detection. The values are expressed as mean±SD (n=3); The values designated by the different letters in the columns are significantly different (p=0.05).
In all arabica coffees the content of caffeic acid was below the detection limit. Similar situation was observed for the extracts of robusta Vietnam decaffeinate, India Cherry and Uganda Bugishu. Ferulic acid was found at very similar level (12.5 - 12.8 mg·L-1) only in robusta coffees except Vietnam decaffeinated. Thus, during the decaffeination process (which is performed prior to the roasting), losses of ferulic acid were occurred. The content of that acid at 5.58 mg·g-1 level was reported in green arabica from Mexico.19
Reducing power of coffee extracts
It is assumed that the antioxidant activity is equal to the reducing capacity of a sample and the level of reducing power is mainly contributed by the hydroxyl groups linked with a benzene ring in an antioxidant molecule. The obtained results for both assays are presented in Figure 1. The Folin-Ciocalteu method is the one adopted in almost all the published works regarding screening of natural antioxidants and the obtained results are usually expressed as gallic acid equivalent (GAE.).1,9 However, to standardize the results from various studies, the trolox equivalent (TRE) has also been used.1,10
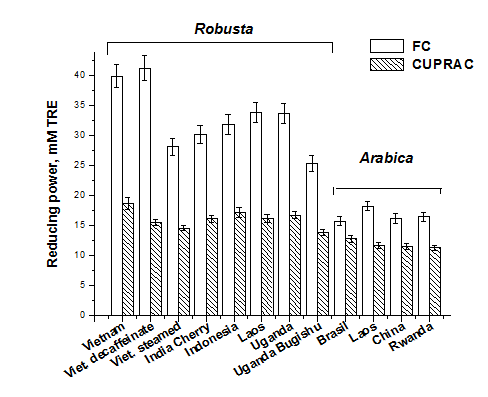
The ability of the studied robusta green coffee to reduce FC reagent was significantly higher than arabica type (Figure 1). This different behavior is due to the phenolic components present in these extracts (Table 2). The highest value was obtained for Vietnam coffee, both caffeinate and decaffeinate (39.90 and 41.3 mM TRE), respectively). The same type of coffee, but steamed at elevated temperature and pressure, exhibited much lower reducing power (28.2 mM TRE). Steaming process is used to remove irritant components and improve coffee aromas creating much more delicate and neutral robusta. Among arabica types, coffee from Laos presented the highest reducing power in FC assay (33.9 mM TRE). Similar order of green coffee reducing power was observed employing CUPRAC method (Figure 1). The highest value was observed for Vietnam coffee (18.7 mM TRE) and the lowest for all arabica types (11.3-12.8 mM TRE).
Coffee |
DPPH |
FIC |
|
% Scavenging |
TRE (mM) |
% |
|
Robusta |
|||
Vietnam |
7.2±0.31a |
4.02±0.16a |
51.7±2.01a |
Vietnam decaffeinate |
10.3±0.41b |
4.50±0.23b |
57.6±2.80b |
Vietnam steamed |
5.9±0.24c |
3.58±0.18c |
48.3 ±1.93c |
India Cherry |
20.1±0.80d |
8.21±0.39d |
52.0±2.57a |
Indonesia |
10.5±0.42b |
5.17±0.25e |
31.2 ±1.53d |
Laos |
21.4±0.86d |
8.66±0.36f |
51.7±2.07a |
Uganda |
5.5±0.86c |
3.47±0.16c |
49.8±2.29a |
Uganda Bugishu |
2.2±0.09e |
2.45±0.12g |
61.7±3.03e |
Arabica |
|||
Brasil |
2.1±0.08e |
2.45±0.10g |
60.2±2.93e |
Laos |
2.2±0.09e |
2.41±0.10g |
63.2±3.14e |
China |
1.2±0.05f |
2.18±0.14g |
51.5±2.01a |
Ruanda |
2.9±0.12g |
2.71±0.12g |
70.7±3.48f |
Table 2 Antioxidant activity of green coffee infusions measured by DPPH and metal chelating assays
The values are expressed as mean±SD (n=3); The values designated by the different letters in the columns are significantly different (p=0.05).
In order to correlate the used methods for evaluation of reducing power, a linear regression model was used. The correlation between FC and CUPRAC assays, both express as trolox equivalent, for was not highly significant (R2 = 0.898). Although both methods are based on the redox properties of the sample components, but with different reduction potentials, different kinetics and experimental conditions. Generally, determination of the reducing power using FC assay is based on the level of a compound in reducing the Folin-Ciocalteau reagent (phosphotungstic and phosphomolybdic acids mixture) to produce blue oxides of tungsten and molybdene under an alkaline conditions. This assay is often named as the total of phenolic, however, the used reagent is not specific only for phenolic compounds. Several non-phenolic compounds including proteins, amino acids, thiols, vitamins and inorganic ions, which are common in plants, are reactive toward the FC reagent.20 CUPRAC method is based on reduction of Cu(II) to Cu(I) at neutral pH by reductants (antioxidants) present in a sample utilizing the copper(II)-neocuproine reagent as the chromogenic oxidant. Simple sugars and citric acid are not oxidized with the CUPRAC reagent.16
DPPH radical scavenging activity
The kinetic curves of scavenged DPPH radical by studied green coffee infusions are shown on Figure 2. A fast and remarkable decrease in the absorbance of DPPH• was observed during the first few minutes of the reaction, particularly for robusta coffees, followed by slow subsequent disappearance of the reagent. This fast step essentially refers to the electron-transfer process from -OH group in phenolic molecule to DPPH radical and latter kinetic reflects the remaining activity of the oxidation-degradation products.21 For arabica types only a slow decay of DPPH• absorbance was observed.
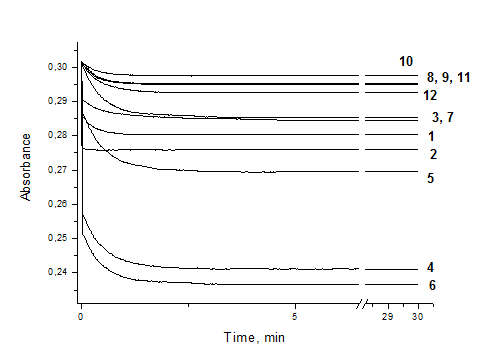
DPPH assay is extensively used to test the ability of compounds to act as free radical scavengers or hydrogen donors, and to evaluate antioxidant capacity of foods.22,23 The obtained results have been presented in many ways. The majority of studies regarding the antioxidant activity of coffee brews express the results in terms of the reduction percentage of the DPPH• solution, referred also as percent of inhibition or quenching.24-26 In some papers the parameter IC50 (the antioxidant or sample concentration necessary to decrease the initial DPPH• concentration by 50%) is preferred.27 However, this parameter highly depends on the reaction time and the initial DPPH• concentration.23 The recommendation was given for using both kinetic and fixed endpoints combination to provide comprehensive information about antioxidant potential.28 The results were also expressed as trolox equivalent (µmol of trolox necessary to provide the same antioxidant activity as a gram of the sample).16
In our study the DPPH radical scavenging activity for green coffee infusions were expressed as the reduction percentage of the DPPH• solution after 30 min of reaction and also with comparison to the changes induced by trolox as a reference compound (express as trolox equivalent, TRE). The obtained results are presented in Table 3. Robusta coffee extracts exhibited higher capacity to scavenge DPPH radical than Arabica types. Comparing different prepared green coffee from Vietnam, it can be seen that decaffeinate coffee presented a greater scavenging activity, followed by raw and steamed coffee.
Coffee |
FC Index |
CUPRAC Index |
DPPH Index |
Chelation Index |
AOX Index |
Robusta |
|||||
Vietnam |
96.6 |
100 |
46.4 |
73.1 |
79 |
Vietnam decaffeinate |
100 |
82.9 |
52 |
81.5 |
79.1 |
Vietnam steamed |
68.3 |
78.1 |
41.3 |
68.3 |
64 |
India Cherry |
73.1 |
86.6 |
94.8 |
73.6 |
82 |
Indonesia |
77.2 |
92 |
59.7 |
44.1 |
68.3 |
Laos |
82.1 |
86.6 |
100 |
73.1 |
85.5 |
Uganda |
81.6 |
89.8 |
40.1 |
70.4 |
70.5 |
Uganda Bugishu |
61.5 |
74.3 |
28.3 |
87.2 |
62.8 |
Arabica |
|||||
Brasil |
38.3 |
68.4 |
28.3 |
85.1 |
55 |
Laos |
44.3 |
62.6 |
27.8 |
89.4 |
56 |
China |
39.2 |
62 |
25.2 |
72.8 |
49.8 |
Ruanda |
40 |
60.4 |
31.3 |
100 |
57.9 |
Table 3 Antioxidant index for green coffee infusions
Metal chelating activity
The ferrous-ion-chelating (FIC) assay measures the ability of secondary antioxidants to chelate metal ions as these antioxidants act indirectly by preventing the formation of free radicals through the Fenton`s reaction. The antioxidant activity of the studied green coffee infusions measured by metal chelating assay is presented in Table 2. Arabica green coffee brews had a little stronger chelating activity (52-71%) on Fe2+ ions than robusta types (31-62%). Tang et al.18 reported that green tea extract (containing 86% of dietary tea catechins) possessed limited chelating effects.
Antioxidative index
In order to correlate the obtained results using different assays an antioxidant index (AOX) was calculated using the procedure described by Seeram et al.29 For all assays appropriate index was calculated as a percentage of average antioxidant capacities of a given sample compared to the highest one (sample score/best score x 100). Then AOX index was obtained for each sample as the sum of the individual indexes divided by the number of tests. The obtained results are presented in Table 3.
Among studied extracts, robusta green coffee from Laos had the most potent antioxidant capacity, followed by India Cherry. Green coffee from Laos contains the highest content of chlorogenic acid (Table 2), As it was expected, for arabica coffees lower values of the antioxidant index were obtained, the lowest for coffee from China.
The results of this research have provided an insight into the differences in both antioxidant properties and the concentrations of the main phenolic acids in green coffee infusions, both robusta and arabica types. The combination of different methods (reducing power, free radical scavenging, chelation assay) gave an idea of their protective potential against oxidative stress.
It should be emphasized that the assays employed in this study are strictly based on chemical reactions in vitro and they bear no similarity to biological systems. However, spectrophotometric methods for the evaluation of antioxidant activity of food samples are still the most widely used. With these kinds of methods, reagents are easy to get, results are given quickly and experiments are convenient. It is great of importance to study in vivo whether there is a correlation between the intake of high potent antioxidants and the level of oxidative stress.This article was financially supported (to MJS) within the project "Engineer of the Future. Improving the didactic potential of the Poznan University of Technology" - POKL.04.03.00-00-259/12, implemented within the Human Capital Operational Programme, co-financed by the European Union within the European Social Fund. Authors would like to thank Strauss Café Poland for the samples.
The author declares no conflict fo interest.

©2016 Sentkowska, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.