MOJ
eISSN: 2381-182X


Research Article Volume 3 Issue 2
Nutrition and Food Science, University of Valencia, Spain
Correspondence: A Frígola, Nutrition and Food Science, University of Valencia, Avda, Vicent Andrés Estellés, s/n 46100 Burjassot, València, Spain, Tel 00-34-963544955, Fax 00-34-963544954
Received: October 11, 2016 | Published: November 22, 2016
Citation: Köstekli M, Özdzikicierler O, Cortes C, et al. Role of potassium permanganate ethylene on physicochemical properties, during storage of five different tomato cultivars. MOJ Food Process Technol. 2016;3(2):281?289. DOI: 10.15406/mojfpt.2016.03.00069
Although tomatoes should be ripened at room temperature to obtain the best flavour, the majority of consumers who preserve food at home store tomatoes in the fridge for 4-7days and some people even keep tomatoes for up to 3weeks in these conditions. In the present work the effects of storage time and potassium permanganate ethylene absorber sachets under refrigerated conditions (similar to those used by consumers who preserve tomatoes at home) on physicochemical properties, ascorbic acid content and antioxidant capacity of five tomato cultivars (Cherry, Cherry pera, Rama, Raf and Pera) were studied. After 25days storage at 4±2°C an increase in fruit pH was observed for the five cultivars without and with ethylene absorber sachets. The increase in pH was paralleled by a decrease in titratable acidity. The degradation of ascorbic acid during storage time with and without ethylene absorber sachets followed a first-order kinetic model. In general, the percent loss of ascorbic acid and antioxidant capacity measured with TEAC and ORAC methods was significantly lower in tomatoes stored with ethylene absorber sachets as compared to tomatoes preserved without these. In addition, a positive correlation was found between ORAC and TEAC methods for the determination of antioxidant capacity in all tomato cultivars.
Keywords: tomato, ethylene absorber sachets, potassium permanganate, ascorbic acid, total antioxidant capacity
Increased intake of fruits and vegetables has been associated with reduced incidence of some types of cancer and heart diseases1 and it is attributed to their significant levels of biologically active components with physiological and biochemical functions which benefit human health,2 such as antioxidants that are against free radicals.3
The tomato (Lycopersicon esculentum) is the most widely consumed fresh vegetable in the industrialized world and it is found at the base of the pyramid of the Mediterranean diet.4 It is also widely used for the production of derived products such as juice, purées, ketchup or soups.5,6 The tomato is known to be beneficial for health, especially with regard to the development of chronic degenerative diseases mainly due to its high content in health-related compounds such as ascorbic acid, carotenoids (in particular, lycopene), and phenolic compounds thus having antioxidant capacity.7 Moreover, ascorbic acid and antioxidant capacity can be useful quality indicators in fruits and vegetables because they are sensitive parameters providing an indication of cultivar, environmental conditions, use of production techniques and storage conditions after postharvesting and how it affects to organoleptic characteristics or nutritional components.8-10
During storage, tomato fruit characteristically follow a climacteric ripening pattern which is controlled by ethylene,11 involving a wide range of physical, biochemical and physiological changes, which start in fruit of the plant and follow after detachment from it, and these changes determine the fruit nutritional and quality attributes.12,13
Mostly, technologies during tomato storage are focused to control biosynthesis and ethylene action for delaying the development of negative changes and to extend shelf life maintaining the best quality until consumption.14 Generally, cold storage seems beneficial in delaying the fruit ripening due to inhibition of ethylene production.15 However, edible coatings, heat treatment, controlled and modified atmosphere storage, exogenous application of calcium, selenium, aminoethoxyvinylglycine and 1-Methylcyclopropene (1-MCP) resulted in a limited success in extending shelf life, maintaining fruit quality and minimizing postharvest losses of fruits.16-18
It is reported that potassium permanganate is able to remove the exogenous ethylene from atmosphere, which played a central role in tomato fruit ripening by absorbing and oxidizing it to carbon dioxide and water, thus increasing concentration of carbon dioxide and blocked the synthesis of endogenous ethylene, which is said to be essential for control of ripening as its synthesis is believed to be essential for many plant developmental processes including ripening.13,19 It is followed from the literature that ethylene accumulation and its harmful effect on vegetables during post-harvest storage are obviously important for both nutritional and organoleptic quality of vegetables.
Some previous publications have studied the effect of ethylene absorbers, including potassium permanganate, on different cultivars at different maturity stages using the recommended conditions. However, most of the consumers who preserve foods at home store tomatoes in the fridge (4±2 °C) for 4-7 days and some people keep tomatoes for up to 3 weeks in these conditions. The aim of this work is to study the effect of potassium permanganate included absorber sachets on physicochemical properties, ascorbic acid content and antioxidant capacity of different cultivars of tomatoes during refrigerated storage in similar conditions to those used for consumers who preserve tomatoes at home.
Plant material
One hundred and eighty five samples, belonging to five cultivars of tomatoes (Cherry, Cherry pera, Rama, Raf and Pera) from Almeria (Spain), grown in greenhouse conditions were used in this study. Tomatoes were selected at the same maturity stage at time of harvest. Five stages were established according to a rank of maturity, which were defined in accordance to Casierra-Posada & Aguilar-Avendaño20 using 5 color ratios: stage 1) fruits were completely green; stage 2) 75% green: 25% red; stage 3) 50% green: 50% red; stage 4) 25% green: 75% red; and stage 5) fruits were full-ripe and red. All tomato samples arrived to laboratory less than 24 h after harvesting at maturity stage 3 and the quality parameters were tested immediately after harvest. These were divided in two groups. One group was stored with ethylene absorber material and other without ethylene absorber material. Both were stored under refrigerated conditions (4±2 °C) and darkness storage area. Three tomatoes were randomly selected from each tomato sample and from each group (without and with ethylene absorber sachets) for analysis. Tomatoes were taken from the storage, weighed, mixed, processed with a juice machine and then homogenised. Then the juice was stored in glass bottles in the same dark refrigerated area.
Materials and reagents
Ethanol, methanol, and sodium chloride (special grade) were purchased from J.T. Baker (Deventer, The Netherlands). Potassium hydroxide and sodium sulphate (Scharlab, Barcelona, Spain), L(+)-ascorbic acid (Merck, Darmstadt, Germany), Trolox® (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), ABTS (2,2'-azinobis(3-ethylbenzothiazoline 6-sulfonate)). Sodium and disodium phosphate, magnesium hydroxide carbonate (40-45%), and 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH) were purchased from Panreac (Barcelona, Spain). Potassium permanganate ehylene absorber sachets (26 x 30 cm) consisted on a sealed sachet containing 20 grams of zeolite pellets impregnated with potassium permanganate. The sachet is housed in a plastic cartridge for use in refrigerator storage bins to extend the storage life of product. The cartridge removes harmful ethylene gas produced from fruits and vegetables keeping the product fresh for longer periods of time. It is composed of 94% Clinoptilolite, a natural occurring zeolite, and 6% potassium permanganate (Keepfresh® sheet ethylene absorber post-harvest treatment of vegetables).
Methods
Physicochemical parameters:pH, titrable acidity and total soluble solids (TSS) analysis were performed according to AOAC.20 The relationship between total soluble solids and titrable acidity known as maturity index was determined in accord to Casierra-Posada & Aguilar-Avendaño.21 Weight loss was determined in accord to Ferreira et al.22 by the percent relation between initial and final weight of the fruit, every three days, from the harvest day to the end of the storage period. To measure the juice content of a fruit, a representative sample of fruit is taken and then the juice extracted. The juice volume is related to the original mass of juice, which is proportional to its maturity.23
Determination of ascorbic acid: A Metrohm 746 VA trace analyzer (Herisau, Switzerland) equipped with a Metrohm 747 VA stand was used. The working electrode was a Metrohm multimode electrode operated in the dropping mercury mode. A platinum wire counter electrode and a saturated calomel reference electrode were used. Tomato juice (5 mL) was diluted to 25 mL with the extraction solution (oxalic acid 1%, w/v, trichloroacetic acid 2%, w/v, sodium sulfate 1%, w/v). After vigorous shaking, the solution was filtered through a folded filter (Whatman 1). Oxalic acid (9.5 mL) 1% (w/v) and 2 mL of acetic acid/sodium acetate 2 M buffer (pH=4.8) were added to an aliquot of 0.5 mL of filtrate, and the solution was transferred to the polarographic cell. The following instrumental conditions were applied: DP50, mode DME, drop size 2, drop time 1 s, scan rate 10 mVs-1, initial potential -0.10 V. Determinations were carried out by using the peak heights and standard additions method.24
Analysis of total antioxidant capacity
Trolox Equivalent Antioxidant Capacity (TEAC) Assay: The method used was described by Re et al.25 based on the capacity of a sample to inhibit the 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radical compared with a reference antioxidant standard (Trolox®). The radical was generated using 440 μL of potassium persulfate (140 mM). The solution was diluted with ethanol until an absorbance of 0.70 was reached at 734 nm. Once the radical was formed, 2 mL of ABTS•+ was mixed with 100 μL of appropriately diluted sample and the absorbance was measured at 734 nm for 20 min in a Perkin Elmer UV/Vis Lambda 2 spectrophotometer (Perkin-Elmer, Jügesheim, Germany) in accordance with Barba et al.24
Oxygen Radical Absorbance Capacity (ORAC) Assay: The oxygen radical absorbance capacity (ORAC) assay used, with fluorescein (Sigma-Aldrich, Steinheim, Germany) as the “fluorescent probe,” was that described by Ou et al.26 The automated ORAC assay was carried out on a Wallac 1420 VICTOR2 multilabel counter (Perkin-Elmer, USA) with fluorescence filters, for an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The measurements were made in plates with 96 white flatbottom wells (Sero-Wel, Bibby Sterilin Ltd., Stone, UK). The reaction was performed at 37 °C as the reaction was started by thermal decomposition of AAPH in 75 mM phosphate buffer (pH 7.0). The final reaction tested and the concentrations of the different reagents were determined following Barba et al.24
Degradation kinetic studies
According to Lavelli & Giovanelli27 in their study using tomato-derived products, the ascorbic acid degradation during storage followed a first-order reaction; this kinetic type was expressed by the following equations:
Ct = C0 exp(-kt) (1)
t1/2 = -ln 0.5/k (2)
where C0 is the initial ascorbic acid content, Ct is the ascorbic acid after a certain storage time t (days) at the given storage temperature, k is the first-order kinetic constant and t1/2 is the half-life time.
Statistical analysis
The measurements were taken in triplicate. Significant differences between the results were calculated by analyses of variance (ANOVA) and the possible interactions between the parameters. An LSD test was applied to indicate the samples between which there were differences. A multiple regression analysis was performed to study the influence of different factors on a given parameter. The correlations between a pair of variables also were studied using a Pearson test. In the shelf-life study, an ANOVA of three factors was applied to the physicochemical parameters, ascorbic acid and antioxidant capacity in order to detect differences between the effect of cultivar (Cherry, Cherry pera, Rama, Raf and Pera), the ethylene absorber sachet (without or with), and the storage time (0, 3, 6, 11, 18, and 25 days). For the parameters with more than two levels (storage time) with significant differences (p<0.05) a variance analysis and LSD test (p<0.05) was applied in order to identify between which levels differences existed. All statistical analyses were performed using SPSS 20.0® (SPSS Inc., Chicago, USA).
The effect of refrigerated storage and the potassium permanganate ethylene absorber sachets on ascorbic acid content, physicochemical properties and antioxidant capacity of five tomato cultivars was evaluated in the present study.
Effect of cultivar, storage time and ethylene absorber sachets on physicochemical parameters
The results obtained for titrable acidity (g citric/100 g) were 0.18±0.01, 0.21±0.01, 0.30±0.01, 0.31±0.01 and 0.33±0.01 for Pera, Rama, Raf, Cherry and Cherry pera cultivars, respectively. After 25 days storage at 4 °C, titrable acidity was decreased for all the cultivars without (15-45%) and with (33-56%) ethylene absorber sachets. As can be observed, the changes were more severe in samples with ethylene absorber sachets compared to samples without sachets. This decrease can be attributed to the use of KMnO4, that contributed to an increase in the CO2 concentration as ethylene is degraded into CO2 and water by the action of KMnO4.28 This CO2 accumulated in the fruit tissue and after dissolving formed carbonic acid, causing acidiosis.29 Sammi & Masud30,31 also found a decrease in titrable acidity of tomato during ripening when they used sponge cuttings that dipped in saturated potassium permanganate solution. pH values ranged from 4.00±0.03 (Cherry, Cherry pera and Raf cultivars) to 4.37±0.02 (Rama and Pera cultivars). An increase in pH values was found after 25 days storage at 4 °C for all the samples studied. This increase was more intense for the tomatoes with ethylene absorber sachets (5-9%) in comparison to the samples without sachets (4-8%). The results obtained in the present study were in accord to those found by Anthon et al.32 These authors attributed the rise in pH and decrease in titrable acidity mainly due to a decrease in acid concentrations of the tomato with maturity. The predominant acid in tomatoes is citric acid, so citric acid levels declined with maturity in all five tomato cultivars analysed in the present study.
Total soluble solids were 4.00±0.01, 5.00±0.01, 5.90±0.01, 6.00±0.01 and 6.50±0.01 for Rama, Pera, Cherry pera, Raf, and Cherry cultivars, respectively. After 25 days storage at 4 °C, a significant decrease in TSS was obtained for Cherry cultivar without and with ethylene absorber sachets (-2%) and Cherry pera with (6%) and without (-23%) ethylene absorber sachets, however a significant increase was obtained for Rama with (10%) and without (13%) ethylene absorber sachets, as well as for Pera cultivars with (5%) and without (9%) sachets. In addition, a significant increase (6%) was found in TSS in Raf samples after 25 days storage at 4 °C while the opposite trend was obtained in the Raf tomatoes with ethylene absorber sachets (-5%). Changes in TSS during storage are correlated with hydrolytic changes in starch concentration during ripening in post-harvest period. In tomatoes, conversion of starch to sugar is an important index of ripening.33 Different studies available in the present literature have reported contradictory results about the behavior of TSS in tomatoes. Sammi & Masud30,31 reported an increase in TSS during the ripening period of tomatoes. However other authors reported a decrease in TSS21,34 or even non-significant changes.35,36
Initial and final weights (g) of Cherry, Cherry pera, Rama, Raf and Pera cultivars during 25 days storage at 4 °C are shown in Figure 1. Significant differences (p<0.05) in weight were observed at the end of the storage for tomatoes without ethylene absorber sachets, from -3% (Raf) to 19% (Pera) and for tomatoes with ethylene absorber sachets, from -5% (Rama) to 10% (Pera).
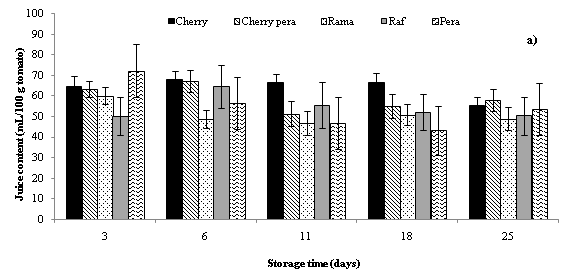
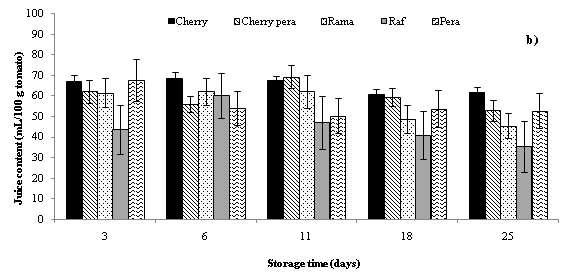
The highest decrease injuice content (mL/100 g tomato) was found in Cherry Pera cultivars, since the juice volume of 100 g Cherry pera tomatoes decreased for tomatoes without ethylene absorber sachets (from 63.4 to 41.5 mL) and for tomatoes with sachets (from 62.2 to 38.8 mL) (Figure 2). Regarding to Raf cultivar, volume of juice obtained was increased between days 5 and 10. After the day 10 the juice volume of the Raf sample was decreased slightly to the end of the storage period as well as the other cultivars. Some previous works indicate that the potassium permanganate is said to be an ethylene degrading chemical which degrades ethylene into water and carbon dioxide. Water accumulated in storage atmosphere created a high humid environment retarding transpiration and water loss.28,31,36 In addition, increase in concentration of carbon dioxide blocks the synthesis of endogenous ethylene.37 In the present study the ambient temperature of the storage area changed between 3-5 °C. The temperature factor over weight loss during storage is studied and explained in some previous studies in the published literature. Some previous studies reported in the published literature have demonstrated that the weight loss of tomatoes stored at room temperature was significantly higher than low temperature stored tomatoes.36,38 So, they recommended refrigerated conditions in order to storage better tomatoes.
Effect of storage time and ethylene absorber sachets on ascorbic acid content
The ascorbic acid content in the tomato cultivars ranged from 10.5 to 22.8 mg/100 g. These values were in the range of those previously reported by Guil-Guerrero & Rebolloso-Fuentes39 when they studied eight tomato cultivars (Cherry, Cherry Pera, Daniela Lara Vida, Lido, Pera, Racimo, Raf and Rambo). Analysis of the variation in the ascorbic acid content of the five tomato cultivars at the beginning of the storage, showed significant differences (p<0.05) among all the cultivars. The highest ascorbic acid content was found in Cherry cultivar (22.8±0.7 mg/100 g), followed by Cherry pera (17.3±0.5 mg/100 g), Pera (14.4±0.3 mg/100 g), Rama (11.7±0.4 mg/100 g) and Raf (10.5±0.6 mg/100 g). George et al. [40] also observed higher ascorbic acid contents in pulp (32.4 mg/100 g) and skin (56.0 mg/100 g) in 818 Cherry cultivar, and they indicated Cherry as a possible recommended cultivar for processing and to increase nutritional value in educational programs due to higher contents in ascorbic acid. However, Raffo et al.41 observed lower vitamin C content in Cherry cultivar 5.5 mg/100 g, this value is lower than the obtained in the present study. In food composition tables, vitamin C contents in tomatoes are in the range of 19-36 mg/100 g. A possible reason for the differences obtained in ascorbic acid content for the same cultivar can be explained for the incident light in tomato at the end of harvesting, temperature conditions during pre-harvesting, harvesting time and post-harvesting.
The three-way ANOVA showed that cultivar, the use of ethylene absorber sachets and storage time had a significant influence (p<0.05) on the ascorbic acid of the tomatoes analyzed in the present study, obtaining significant interactions. During 25 days storage at 4 °C, ascorbic acid decreased significantly (p<0.05) for all cultivars except Raf tomato when it was stored without ethylene absorber sachets and Rama cultivar with sachets. Not all the samples showed a regular behaviour that makes it possible to easily identify it with a given model. This is due to the fact that tomatoes are a living material and therefore subject to physiological changes that are a function of several factors (origin, cultivar, developmental stage, temperature, etc.).
However, a model of the behaviour of ascorbic acid content as a function of storage time, which clearly obeys an exponential negative law, was observed for the majority of the samples. Only Raf cultivar stored without ethylene absorber sachets and Rama cultivar stored with sachets, which gave a graph with a linear tendency, seemed to behave differently from the others.
However, it appeared to be reasonable to obtain a similar behaviour model in all of the cases, rather than a different one for each sample. This fact was later confirmed when, in adjusting the exponential model, the correlation coefficients obtained were statistically significant in all the samples.
Therefore, variations in the ascorbic acid content of tomatoes during storage in the previously mentioned conditions obey an exponential function. In Table 1 the equations obtained for each of the samples and the corresponding correlation coefficients are reported. The loss of ascorbic acid during storage may have been the result of oxidation of ascorbic acid due to its sensitivity to O2, light and temperature as previously reported Nagy.42 In addition, at the end of the storage, both groups of Cherry cultivars (with and without sachets) showed the highest ascorbic acid content (20.38 and 13.90 mg/100 g, respectively).
A comparison of the slopes of the degradation curves showed a behaviour that depends on the cultivar and the presence of ethylene absorber sachets. Overall, all the samples with ethylene absorber sachets showed a higher content in ascorbic acid content at the end of the storage than the samples without ethylene absorbers except Raf cultivar (with sachets 7.2±0.2 mg/100 g, without sachets 10±0.1 mg/100 g). It is stated that potassium permanganate absorbs ethylene and degradates it to CO2 and water which results in an increase of CO2 content at storage atmosphere.29 In addition, increase in concentration of carbon dioxide blocks the synthesis of endogenous ethylene37 which is a ripening gas for fruits and vegetables. From this point of view it is accessible that using ethylene absorber laminas for Raf cultivar caused a slowed down ripening period which ended with lower ascorbic acid content than without laminas group.
Tomato |
C0 |
Ka |
t1/2b |
Correlation Coefficient |
Without Ethylene Absorber |
||||
Cherry |
23.44 |
0.017 |
40.77 |
0.918 |
Cherry pera |
17.78 |
0.013 |
53.31 |
0.925 |
Rama |
11.99 |
0.026 |
26.65 |
0.976 |
Raf |
9.85 |
0.005 |
119.51 |
0.968 |
Pera |
13.63 |
0.023 |
30.14 |
0.909 |
With Ethylene Absorber |
||||
Cherry |
23.21 |
0.004 |
154.03 |
0.786 |
Cherry pera |
16.42 |
0.011 |
63.01 |
0.827 |
Rama |
11.67 |
0.006 |
115.52 |
0.906 |
Raf |
10.21 |
0.003 |
231.05 |
0.698 |
Pera |
14.81 |
0.025 |
27.73 |
0.945 |
Table 1 Effect of storage time and potassium permanganate ethylene absorber sachets on k and t1/2 values of ascorbic acid degradation in tomato cultivars
aRate constant
bHalf-life
Moreover, according to statistical analysis it is obvious that there was a negative significant correlation (p<0.05) between ascorbic acid and pH (r = -0.504, p<0.05). As it was reported previously, Cherry cultivar had the lowest pH values at the end of the storage period. This fact can explain the better preservation of ascorbic acid during storage of this cultivar. In addition a negative correlation (r = -0.628, p<0.05) was found between ascorbic acid and weight loss. This fact can explain the differences in ascorbic acid retention of Raf and Rama cultivars during 25 days storage at 4 °C.
The values of antioxidant capacity of the five tomato cultivars measured with TEAC and ORAC methods are shown in Tables 2 & 3. Results obtained with TEAC method showed that Cherry cultivar had the highest content in the beginning of the study (344.2±51.3 μmol trolox in 100 g) while the lowest values (195.5±23.4 μmol trolox/100 g) were found in the Raf cultivar. TEAC values of tomatoes were in the range of those previously reported by Pellegrini et al.43 & Apak et al.44 when they studied different tomatoes and tomato-derived products. Moreover, the three-way ANOVA analysis showed that cultivar and storage time had a significant influence (p<0.05) on antioxidant capacity of the tomatoes measured with TEAC assay, obtaining significant interactions (p<0.05). During 25 days storage at 4 °C, TEAC values decreased significantly (p<0.05) for all cultivars stored without and with ethylene absorbers sachets.
Storage (Days) |
Cherry |
Cherry Pera |
Rama |
Raf |
Pera |
0 |
344.2±51.3a |
331.8±12.7a |
249.1±22.8abc |
195.5±23.4abc |
240.6±20.9ab |
Without Ethylene Absorber |
|||||
3 |
312.8±28.6abc |
280.5±8.0b |
235.1±3.5ab |
175.9±6.3ab |
246.6±8.3a |
6 |
290.0±11.5abcd |
211.3±15.2cd |
229.6±1.1abc |
169.7±5.0ab |
238.3±7.4a |
11 |
198.4±25.4e |
195.5±3.9de |
191.5±13.8dc |
168.1±6.0ab |
215.2±5.3abc |
18 |
194.9±31.8e |
199.0±7.0de |
177.0±6.0de |
163.0±4.9a |
194.6±23.4abcd |
25 |
191.3±29.6e |
164.9±12.1ef |
137.9±11.5e |
160.9±5.0a |
128.7±6.5e |
With Ethylene Absorber |
|||||
3 |
331.8±30.3a |
254.5±22.7bc |
248.6±1.8a |
196.9±9.7c |
228.1±9.4ab |
6 |
294.9±19.4abcd |
234.0±5.2cd |
225.2±14.3abc |
197.8±12.2bc |
229.3±12.5a |
11 |
273.2±11.6bcd |
216.4±5.9cd |
213.5±5.7abcd |
194.9±10.8abc |
175.9±7.9bcde |
18 |
251.7±27.6cde |
202.0±21.9de |
207.0±14.6bcd |
182.8±15.9abc |
166.1±7.9cde |
25 |
231.8±34.1de |
147.8±24.0e |
147.2±5.7e |
181.2±10.2ab |
156.9±5.9de |
Table 2 Effect of storage time and ethylene absorber sachets on antioxidant capacity (TEAC) of tomato cultivars (μmol trolox/100 g)
a-fDifferent letters in the same column indicate significant statistical differences in function of the storage time
Storage (days) |
Cherry |
Cherry Pera |
Rama |
Raf |
Pera |
0 |
321.3±0.4a |
291.8±1.8a |
192.7±1.5a |
158.5±2.5a |
209.4±0.2a |
Without Ethylene Absorber |
|||||
3 |
328.3±0.5b |
270.2±2.0b |
182.6±1.7b |
151.2±2.0b |
178.2±5.0b |
6 |
329.6±3.2b |
258.1±0.6c |
180.3±0.5b |
140.4±1.3c |
187.9±2.0bc |
11 |
302.7±6.1c |
247.8±1.1d |
178.7±0.1bc |
135.6±0.1d |
182.7±0.5bc |
18 |
304.4±1.8c |
239.7±0.1e |
163.9±0.1d |
137.9±0.1d |
176.9±1.9b |
25 |
285.5±3.6d |
224.8±0.5f |
152.3±1.4e |
129.6±3.8e |
165.6±1.1c |
With Ethylene Absorber |
|||||
3 |
312.3±3.0c |
245.5±0.7d |
185.4±5.0b |
150.2±1.6b |
201.3±4.0d |
6 |
307.3±4.2c |
204.1±2.1g |
182.0±1.7b |
149.1±0.9b |
186.4±3.5bc |
11 |
309.5±5.7c |
200.4±0.6g |
188.3±1.8bf |
146.4±0.4f |
194.8±0.3e |
18 |
304.9±2.9c |
182.3±0.8h |
170.9±1.9g |
142.5±0.8c |
165.3±1.1c |
25 |
305.5±3.2c |
199.8±0.8g |
168.5±1.1g |
132.8±0.6e |
161.5±2.9c |
Table 3 Effect of storage time and ethylene absorber sachets on antioxidant capacity (ORAC) of tomato cultivars (μmol trolox/100 g)
a-hDifferent letters in the same column indicate significant statistical differences in function of the storage time
With regard to ORAC method, the highest values of antioxidant capacity in the beginning of the study were obtained for Cherry cultivar (321.3±0.4 μmol trolox/100 g), followed by Cherry pera, Rama, and Raf while the lowest value was found in Raf cultivar (158.5±2.5 μmol trolox/100 g). ORAC values of tomatoes analysed in the present study were in the range of those previously reported by Ou et al.26 & Apak et al.44 In the present work, the ANOVA analysis showed that cultivar, the use of ethylene absorber sachets and storage time had a significant influence (p<0.05) on the antioxidant capacity (ORAC values) of the tomatoes analyzed in the present study. Cherry and Cherry pera cultivars showed the higher values of total antioxidant capacity (ORAC), followed by Rama and Raf at the end of the storage (day 25). However, Raf cultivar showed the lowest content of antioxidant capacity in both groups (with or without ethylene absorber sachets). Moreover, ORAC values were significantly higher (p<0.05) for Cherry pera tomatoes without ethylene absorber sachets during all the storage period in comparison to samples with sachets. In the present study, it was found a similar trend in antioxidant values of tomato after storage measured with TEAC and ORAC assays. Likewise, a different behavior in antioxidant values was obtained at the end of the storage depending on cultivars and storage with or without ethylene absorber sachets.
When the possible correlation (Pearson test) among physicochemical parameters, ascorbic acid content, and antioxidant capacity (TEAC and ORAC) was studied, it was found that there was a positive correlation between ascorbic acid with TEAC (r = 0.769, p<0.05) and ORAC (r = 0.765, p<0.05). In addition a positive correlation (r = 0.564, p<0.05) was found between TEAC and ORAC methods. Several studies available in the published literature found good correlations between TEAC and ORAC assays. Proteggente et al.45 found a good correlation between the ORAC and TEAC methods for tomato fruit. Barba et al.6 also found good correlations (r = 0.823, p<0.05) upon comparing the antioxidant capacity results obtained with the TEAC and the ORAC method in a vegetables beverage containing tomato as the main ingredient. Taipong et al.46 & Stintzing et al.47 also obtained comparable antioxidant capacity results for guava juice and “cactus pear” juice, respectively with the TEAC and ORAC methods. In addition, Silva et al.48 compared the ORAC and TEAC methods to measure the antioxidant capacity of 15 Brazilian plants from the Amazonian region, finding that there was a moderate correlation between the results found with the two methods (r = 0.551, p<0.01).
In general, findings from the present study revealed that pH values were higher in fruit with potassium permanganate ethylene absorber sachets, while the opposite trend was found for total acidity. The kinetic of degradation of the ascorbic acid content of tomatoes during storage with and without ethylene absorber sachets fits a first-order exponential model. In general, the comparison of the slopes of the degradation curves showed a behavior that depends on the tomato cultivar and the presence of ethylene absorber sachets, observing lower degradation rates for the samples with ethylene absorbers. Thus, it can be concluded that potassium permanganate ethylene absorber sachets can be a good strategy for consumers who preserve foods at home in order to preserve ascorbic and antioxidant capacity of tomatoes during refrigerated storage, being this treatment effective to improve the storage life and quality of tomato fruits.
This research project was supported by the Spanish Ministry of Science and Technology and European Regional Development Funds (AGL2010-22206-C02-01). Köstekli M, & Özdikicierler O hold an Erasmus research grant.
The author declares no conflict of interest.

©2016 Köstekli, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.