Journal of
eISSN: 2373-4310


Research Article Volume 2 Issue 2
1Department of Medicine and Health Sciences, Monash University, Malaysia
2Department of Medicine and Health Sciences, International Medical University, Malaysia
3Sirim Berhad, Malaysia
4Department of Medicine Health and Molecular Sciences, James Cook University, Australia
5Department of Medicine and Health Sciences, Universiti Tunku Abdul Rahman, Malaysia
Correspondence: Uma D Palanisamy, School of Medicine and Health Sciences, Monash University, Jalan Lagoon Selatan, 46150, Sunway Campus, Malaysia, Tel 603-55145840, Fax 603-55146323
Received: October 30, 2014 | Published: June 8, 2015
Citation: Palanisamy UD, Sivanathan M, Subramaniam T, et al. Refining ostrich oil and its stabilization with curcumin. J Nutr Health Food Eng. 2015;2(2):63-69. DOI: 10.15406/jnhfe.2015.02.00051
Ostrich Oil is particularly known for its cosmetic and therapeutic use. Being rich in polyunsaturated fatty acids, it is prone to oxidation causing undesirable changes in peroxide value, color, flavour and odor, which deteriorates its nutritional value. The objective of the study is to establish an efficient separation method to refine ostrich oil and stabilize it with curcumin or the extract of Curcuma longa. The reduction in peroxide value, color intensity, moisture content and odor were used as indicators of the purity of the oil. The modified refining process developed saw a reduction of 75% free fatty acid, 90% peroxide value, 54% moisture content, 60% in color intensity and odor. Curcumin, a natural antioxidant, prevented lipid peroxidation by 56% while vitamin E by only 8%, when tested at the same concentration (0.04%). Curcumin proved to be an effective stabilizer in a 6-month accelerated stability test. This study presents a method to refine ostrich oil efficiently and the ability of a natural antioxidant, curcumin, to stabilize the refined ostrich oil. This simple and straight forward method to refine and stabilize the oil can be adapted to be used for any ratite oils. The use of a natural alternative, curcumin as the anti-oxidant is not only safe but provides further added health benefits.
Keywords: ostrich oil, refining, oxidation, stabilization, anti-oxidant, curcumin
MUFA, mono-unsaturated fatty acids; PUFA, poly unsaturated fatty acids; SFA, saturated fatty acids
The ostrich, a flightless bird of the ratite family (emu, rhea and ostrich) originating in Africa, is the world’s largest bird. Ostrich oil has been used for centuries by the Egyptian, Roman and African cultures for topical relief.1 Ostrich oil penetrates deeply into the skin and, its non-comedogenic property provides moisture for hours without clogging pores. This is due to its high levels of oleic acid2 and as such can be used as a carrier agent in combination with various medicinal or cosmetic ingredients, to deliver them beneath the skin barrier.3 Emu oil, having similar composition as ostrich oil, has anti-inflammatory and skin de-sensitizing properties;4 however, there are limited scientific research data on these properties with ostrich oil. Lipid peroxidation is a major cause of oil degradation. It is a common problem among edible oils and results in sensory changes known as oxidative rancidity.5 Oxidative rancidity is the main reason for oil rejection by consumers. Rancidity causes undesirable chemical change in flavor, color, odor and nutritional value.6 Furthermore, it also promotes formation of free radicals such as hydroxyl and peroxyl radicals, which are associated with mutagenesis, carcinogenesis and aging.7 The level of oxidation indicated by peroxide value should be ideally maintained below 20meq. If used in edible products.8
Refining removes certain minor constituents from crude fats and oils with high yield of purified glycerides. The minor constituents consist of colour pigment, free fatty acids, peroxides, odours and non-fatty materials.9 Free fatty acids formed in ostrich oil further deteriorate into peroxides, which decompose into odorous materials which turns the oil rancid. The purpose of refining is to remove impurities from the ostrich oil without removing or damaging any of the beneficial properties. The conventional method of preserving or reducing the rate of rancidity is by adding tocotrienol or α-tocopherols.10 The addition of vitamin E derivatives carries a risk of exceeding their optimal concentration of 400 to 800mg/day which may cause it to exhibit pro-oxidant properties.11 Plant extracts exhibit a high level of anti-oxidant activity due to their phytochemical content.12,13 Phytochemicals may be as much as 50times more potent than vitamin E and 20times more potent than vitamin C.14,15 Pomegranate peels, an agricultural waste, has been used to stabilize sunflower oil very effectively at concentrations ranging from 800 to 850ppm and was comparable to conventional synthetic antioxidants.16 In addition, it improved resistance of sunflower oil against thermal deterioration changes. Blackcurrant seeds and rosemary extracts, also sources of active antioxidants, are effective soy oil stabilizers.17 Potato peels and sugar beet pulp stabilized both sunflower and soybean oils at 100 and 200ppm respectively.18 The extracts inhibited thermal deterioration of oil by improving its hydrolytic stability, inhibiting double bond conjugation and reducing the loss of polyunsaturated fatty acids. Myricetin, (-)epicatechin, naringin, rutin, morin and quercetin are well known plant phenolics and are more effective than BHA and BHT in retarding the formation of primary and secondary oxidation products of canola oil.19
Curcuma longa, is a local Malaysian spice used in traditional medicine, contains an active principle curcumin (17-bis (4-hydroxy-3-methoxyphenyl)-1,6-hepta-diene-3,5-dione). Curcumin is present at concentrations of up to 5% in Curcuma longa20 and has anti-inflammatory and antioxidant properties. There is an interest in the use of ostrich oil in the cosmetic and nutraceutical industries21 hence a need to develop a simple and economical way to refine and stabilize the oil to prevent peroxidation. The aim of the study was to establish a method of preparing refined ostrich oil and to determine the ability of Curcumin to stabilize refined ostrich oil.
Materials
Frozen ostrich fat was purchased from Jelita Impian Sdn. Bhd. (Seremban, Malaysia). All chemicals used were of analytical grade. Acetic acid and chloroform was obtained from Fisher Chemical, (Waltham, MA, US). Natural earth clay from Natural Bleach Sdn. Bhd.(Subang, Malaysia). Phenolphthalein and curcumin from Fluka Chemika (Buchs, Switzerland) while α-tocopherol, sodium phosphate (dibasic, anhydrous) and sodium thiosulphate was from Merck Chemicals, (Selangor, Malaysia). Ethanol, 95% and starch was from Scharlau chemicals (Barcelona, Spain). Potassium iodide was obtained from Duchefa Biochemie (Haarlem, Netherlands) and sodium hydroxide was purchased from Lab-scan Analytical (Gliwice, Poland).
Preparation of curcuma longa extract
Curcuma longa rhizomes, obtained from Kuala Lumpur, was washed with copious amounts of water followed by distilled water and allowed to air dry at room temperature. It was placed in an oven (Oven Binder, 0-250 deg C) at 40○C for 24hours, after which it was powderized using a Warring blender to a ground sample size of 0.2 mm. Absolute ethanol (analytical grade) at 1:10 (w/v) was added to the powderized samples and extraction was carried out at room temperature for 24hours in an orbital shaker (Protech Model SI-50D). The suspension thus obtained was filtered using a 114 What man filter paper and filtrate collected. The solvent filtrate was concentrated to dryness using a rotary evaporator (Buchi) and kept at 0°C until required.
Refining ostrich oil
Frozen ostrich visceral and adipose fat was obtained commercially. Refining ostrich oil involved cleaning, rendering, fat separation, bleaching and stabilization (Figure 1). The frozen fat was cleaned manually with 1g/100mL (w/v) of distilled water to remove blood, skin, dirt and other matter, after which it was cut into small pieces. A rendering process was followed where pieces of cut frozen fat (250g) was placed in a microwave at 1100 W for 30-45minutes, at 5min intervals (Sharp). The resultant cloudy liquid was separated by centrifugation at 11000rpm for 15min (Sigma 3K30). Two fractions were obtained; the upper clearer fraction which was used for further studies and the lower cloudy fraction. A bleaching technique, previously established in our laborator,22 was followed to remove the peroxides, metals, colour pigments and odour from the crude oil fraction. Natural earth clay (10%) was added to the oil, agitated for 1hr. at 150°C in a nitrogen vacuum trap. The refined oil thus obtained was separated from natural earth clay by centrifugation at 11000rpm for 40 min followed by decantation. Extraction yields’ at each stage of the refining process was calculated. Stabilization of the oil to prevent rancidity and improve shelf-life was later carried out using curcumin and Curcuma longa extracts. The entire process of ostrich oil refinement and stabilization was carried out a number of times using different frozen fat batches and the following analysis was carried out on three batches of the refined oil.
Analysis of ostrich oil
Fatty acid composition: The crude, refined, olein and stearin fractions of ostrich oil (3 samples each) were sent to AOTD (Advanced Oleo chemical Technology Division, MPOB, Selangor) for the determination of its fatty acid composition using the GLC system following the AOCS method.23 A Shimadzu GC-17A Gas Chromatogram was used, equipped with a capillary column BPX 70(30m×0.25m×0.25μm) and FID detector. The column temperature was maintained at 120°C with an increment of 3°C per minute for 57minutes whereas the injector and detector temperatures were at 260°C and 280°C respectively. All samples were analysed in triplicates.
Moisture content: The AOCS method for moisture content determination was followed.24 In a pre-weighed clean and dry beaker, 3 samples of 5g of ostrich oil were heated using a hot plate (Fischer Scientific). End point was determined on the cessation of rising steam bubbles and absence of foam. The sample was heated until incipient smoking was observed; it was cooled to room temperature in a desiccator and weighed. The moisture content was calculated as:% of moisture=Loss in mass, g/Initial mass of sample, g x 100%. The above was repeated several times and results obtained are an average of at least three analysis.
Color intensity: The characteristic yellow color of ostrich oil was determined using a color spectrophotometer (Cary 50 Bio UV visible). Ostrich oil was placed in a cuvette and its absorbance recorded at 425nm.This method was used to determine the percentage of yellow color reduction in the purified oil compared to its crude.25 Three samples of both the purified and crude samples were analysed. All samples were analysed in triplicates.
Peroxide value determination: The AOCS method26 for peroxide determination was followed. To an acetic-acid chloroform solution (3:230; v/v, mL), 5g of ostrich oil was stirred in until completely dissolved. Saturated potassium iodide (0.5mL) was added drop wisein 1min while stirring followed by the addition of distilled water (30mL) and a few drops of starch. The solution was then titrated against 0.01N sodium thiosulphate with constant agitation until the blue gray color disappeared. The titration point was recorded and expressed in (meq) O2/kg oil; the experiment was carried out in triplicates.
Free fatty acid determination: Total free fatty acid was determined using a titration method described by Saad et al.,27 To a solution of hot neutralized alcohol (75mL), 7g oil and 2ml phenolphthalein was added. The mixture was titrated against 0.1M potassium hydroxide solution with continuous shaking until a permanent pink colour appeared and persisted for 30seconds. The acid value is defined as the number of mg of potassium hydroxide required to neutralize the fatty acids contained in 1g of the fat. The determination was performed in triplicates.
Effect of curcuma longa extract and curcumin on the peroxidation of refined ostrich oil
Various concentrations (0.04 to 5%) of Curcuma longa, α-tocopherol and curcumin dissolved in ethanol, were added drop wise to the refined ostrich oil and stirred to ensure complete solubility. The ostrich oil was heated to 80°C for 4hr. with continuous agitation to induce oxidation. Peroxide values were determined prior to heating (control) as well as after the 4hr oxidation, to evaluate the ability of Curcuma longa extract and curcumin to prevent oxidation of refined ostrich oil. α-tocopherol served as the positive control. All tests were analyzed in triplicates.
Effect of curcuma longa extract and curcumin on the stability of refined ostrich oil
An accelerated stability test was conducted by placing the refined oil containing, curcuma longa extract or curcumin in a 70°C incubator for 14days. The peroxide value in the oils was determined on alternatedays. Controls without the addition of the compound/extract were also carried out. The Schaal oven storage stability test of incubating the oil at 70°C for 2weeks is equivalent to a 6 month shelf life study.28 Three samples were analyzed for each test. Statistical significance was determined using the SPSS software version 18 and ANOVA and t-tests were carried out.
Refining ostrich oil and its comparative analysis against the crude oil
Conventionally, rendering is carried out following a wet method where fat is placed on a receptacle and boiling water is added to enable oily liquid to ascend to the surface, which is then separated and washed in a low acid solution. This process causes further oxidation of the unsaturated oil hence raising the peroxide value.29 In this study however, rendering was accomplished using the microwave-assisted extraction technique, which produced yields of 20.5±5.1% (Table 1).
Process |
*Extraction Yield (%) |
@Final Yield (%) |
aRendering |
20.0 ± 5.1 |
- |
bFat Separation; |
80.1 ± 1.8 |
16.1 ± 1.2 |
cBleaching |
86.56 ± 2.1 |
13.9 ± 1.1 |
Table 1 Extraction yields during ostrich oil refining process
*Extraction yield= Fraction (g)/Original (g)*100;
@Final Yield=Fraction (g)/Frozen fat (250g)*100
aOriginal-Frozen fat
bOriginal- Rendered oil
cOriginal-Upper Olein fraction
This fast and efficient unconventional method using microwave energy as a heat source causes selective heating of the matrix over the extract ant. The intermittent temperature applied during the heating does not enhance oxidation while maintaining the peroxide value.30 The benefits of microwave extraction in oil chemistry over conventional heating have been reported recently.31 The fat separation process by centrifugation was used to separate the two layers obtained after rendering, the upper layer was a clear transparent liquid while the lower layer was an opaque yellowish solid at room temperature. The extraction yields of the two fractions were noted (Table 1). Table 2 shows the fatty acid composition of the two layers, the upper layer has a higher olein concentration hence it being a liquid and is termed the olein fraction while the lower solid layer, the stear in fraction has more saturates; palmitic and stearic acids.
Ostrich Oil Fractions |
Crude Ostrich Oil |
Olein Fraction (Upper Layer) |
Stearin Fraction (Lower Layer) |
Refined Olein Fraction (Prior to Stabilisation) |
Peroxide Value (meq.)02/kg oil |
54.0±3.5 |
58.6±3.2 |
52.6±2.9 |
5.0±0.8 |
Free Fatty Acid (wt %) |
2.4±0.2 |
1.4±0.3 |
4.0 ± 0.5 |
0.6±0.3 |
Fatty Acid Composition (%) |
||||
Oleic Acid (Omega 9) |
36.2±1.5 |
37.5±1.9 |
28.6±1.2 |
39.1±2.0 |
Linoleic Acid (Omega 6) |
18.8±0.5 |
23.0±1.3 |
15.1±1.5 |
17.2±1.3 |
Linolenic Acid (Omega 3) |
1.9±0.3 |
2.8±0.7 |
1.7±1.8 |
1.5±0.3 |
Total PUFA |
56.9 ±2.3 |
63.3±3.9 |
45.4±3.5 |
57.8±3.6 |
Stearic Acid |
6.0±0.5 |
5.8±0.9 |
9.2±0.9 |
6.7±0.8 |
Palmitic Acid |
23.8±1.3 |
22.8±1.1 |
36.8±1.9 |
24.2±1.6 |
Other Fatty Acids |
10.9±1.5 |
6.7±3.2 |
4.6±2.0 |
10.7±1.9 |
Total SFA |
40.7±3.3 |
35.3±5.2 |
50.6±4.8 |
41.6±4.3 |
PUFA: SFA |
1.4:1 |
1.8:1 |
01:01.1 |
1.4:1 |
Moisture Content (%) |
1.3±0.5 |
- |
- |
0.6±0.2 |
Yellow Colour (540nm) |
2.8±0.2 |
- |
- |
0.9±0.3 |
Table 2 Peroxide Value, Free fatty acid composition, moisture content and yellow colour of crude ostrich oil, olein fraction, stearin fraction and refined ostrich oil
The content of free fatty acids remaining in the olein fraction was only 1.4% (42% reduction) while that in the stearin fraction was 4.0% (67% increases). The ratio of Omega 3, 6 and 9 remained relatively the same in crude (1:10:19), olein fraction (1:8:13) and stearin fraction (1:9:17). However, stearic and palmitic acid content in the stearin fraction was far higher than in the olein fraction. The stearin fraction which contains high amounts of saturates contributes to its solid, opaque and yellowish appearance. Its high free fatty acid content as well makes it less favourable for use for therapeutic applications. The olein fractions on the other hand, having a lower concentration of free fatty acids, clear in appearance and with similar omega 3:6:9 ratios was used for further refining using the bleaching technique previously optimised in our laboratory.22 The extraction yield at this stage was about 87% which is equivalent to a final yield of 14.3%. An optimised bleaching technique was established using 10% of natural earth clay at 150°C for 1hr under continues nitrogen flow. Peroxide value and free fatty acid composition of crude ostrich oil, olein, stearin fraction and refined ostrich oil is also shown in Table 2. The refined olein fraction saw a 75% reduction in free fatty acid and a 90% reduction in peroxide value compared to crude ostrich oil. Wang & Lin31 reported a similar reduction in peroxide value in soy oil when bleaching was carried out under Nitrogen stream.31 Furthermore, the ratio of mono-unsaturated fatty acids (MUFA) and poly unsaturated fatty acids (PUFA) over the less favourable saturated fatty acids (SFA) in ostrich oil in both the crude and refined oil remained very closely similar, 1.4:1. Liu et al.,32 when extracting ostrich oil from its fat using supercritical fluid extraction technique as well reported a similar PUFA: SFA ratio.
Analysis of ostrich oil
Moisture content in ostrich oil: Refined ostrich oil showed a 54% reduction in moisture content as shown in Table 2. Reduction in moisture is important to prevent growth of microorganisms, which may cause oil contamination and hence reduce shelf life. Akpan et al.,33 on refining castor oil using the iodometric titration detection method reported a 4.15% in moisture while Salunke & Desai34 using the same oil and detection method reported a content of 5-7%. The moisture content of 0.6% reported in this study is one of the lowest values reported among the oils thus far.
Yellow color in ostrich oil: High intensity of yellow color in oil has been associated with the presence of unwanted free fatty acids, peroxides, pigments and toxins that deteriorates the properties of the oil. Pigments have been reported to be unstable in heat, light, oxygen and highly unsaturated molecules, which could further contribute towards the instability of the oil.35 The yellow color of refined ostrich oil was determined and compared against crude ostrich oil. A 60% reduction in yellow intensity was observed after the oil was refined (Table 2). This reduction in color will further improve the oil stability and its appearance. Laada & Saowanee36 reported the use of perlite to remove dark colorants when refining used palm oil.
Stabilization of refined ostrich oil
Effect of curcuma longa extract and curcumin on the oxidation of refined ostrich oil:
Polyunsaturated fatty acids are known to be easily oxidized and are therefore normally enriched with antioxidants such as α-tocopherols and vitamin A.8 In this study, we attempted to stabilize refined ostrich oil using curcuma longa extract and Curcumin, which are known for their high antioxidant activity. The ability of Curcuma extract and Curcumin in inhibiting lipid peroxidation of the oil, will give an indication of its ability to stabilize the oil. Figure 2 shows the percentage inhibition of lipid peroxidation of refined ostrich oil by the extract and Curcumin, while α-tocopherol was used as the positive control.
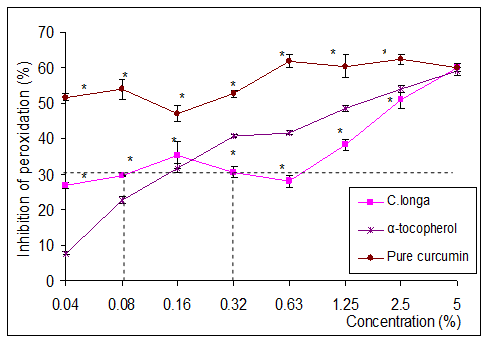
Figure 2 Schaal oven storage stability test performed on refined ostrich oil with various concentration of curcumin for 14 days at 700C.
*Indicates significant difference from control (p < 0.05).
Curcumin, as observed in Figure 2, showed the highest inhibition compared to the Curcuma longa extract and α-tocopherol, at almost all the concentrations tested. It exhibited a constant inhibition of 50-56% over the entire concentration range (0.04-5%) and was significantly different from α-tocopherol at all concentrations except at 5%. Kamat et al.,37 stated that a 30% inhibition of lipid peroxidation is regarded as effective in preventing oil rancidity. Based on this benchmark value, α-tocopherol at 0.1%, curcumin at <0.04% and Curcuma longa extract at concentration ranging from 0.08 to 0.30% are effective in preventing rancidity of ostrich oil. Also observed in this study was that concentrations of curcumin as low as a 0.04% produced similar inhibition of lipid peroxidation to 5% Curcuma longa extract. It has to be mentioned that the active compound in Curcuma longa extract is indeed Curcumin, which constitutes about 5% (w/w) of the extract.20 Aggarwal et al.,38 reported that most of the biological activity related to Curcuma longa is due to Curcumin.
Accelerated stability test on refined ostrich oil
The Schaal oven storage stability test depicts the shelf life of the oil for 6months.28,37 The ability of both Curcumin and Curcuma longa to inhibit peroxidation in refined ostrich oil (Figure 3) (Figure 4) was monitored by measuring its peroxide value. In the control experiment (Refined Ostrich oil without extracts), peroxide values rose up to 180meq. O2/kg oil by day 14. The addition of Curcumin at different concentrations, ranging from 0.5-5.0%, showed a significant reduction in peroxide value (p<0.05). A reduction in peroxide value of almost 90% was observed at 14th day in all the concentration tested compared to the control indicating that Curcumin at concentrations as low as 0.5% can be used to stabilize the refined ostrich oil for a period of 6months.
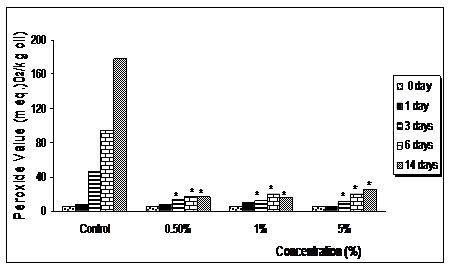
Figure 3 Schaal oven storage stability test performed on refined ostrich oil with various concentration of Curcuma longa for 14 days at 700C.
*Indicates significant difference from control (p < 0.05).
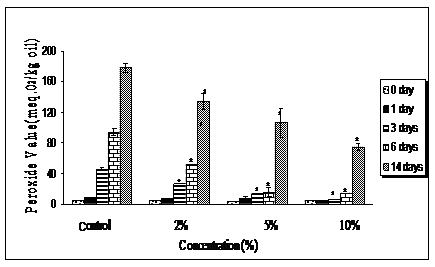
Figure 4 Schaal oven storage stability test performed on refined ostrich oil with various concentration of Curcuma longa for 14 days at 700C.
*Indicates significant difference from control (p < 0.05).
The addition of Curcuma longa at 2-10% concentrations, on the other hand was not as significant as Curcumin (Figure 4). Curcuma longa at 5 and 10% concentrations were effective in preventing peroxide formation up to 6days, which is equivalent to a period of 3months. This study confirms the possible use of Curcumin as an antioxidant agent to stabilize refined ostrich oil.
Method to refine ostrich oil using a dry rendering process, separation by centrifugation and bleaching was established. Owing to the inherent instability of polyunsaturated oils and their inclination to oxidation, many polyunsaturated oils are fortified with antioxidants. Phenolics from plants are a favoured source of antioxidant, not only because of its natural source but due to its additional biological properties. This study has shown that both Curcumin and Curcuma longa extracts are effective in preventing oxidation of the refined ostrich oil. In addition, Curcumin at 0.5% maintains the stability of refined ostrich oil for a period of 6months. Considering the biological properties of Curcumin, its cost and it not being a prooxidant at high concentrations makes it an excellent candidate as a stabilizing agent for polyunsaturated oils.
This research work was supported in part by research grants from the Ministry of Health, Malaysia. JPP-IMR Project ID: 07-018.The authors have declared no conflicts of interest.
Author declares that there is no conflict of interest.

©2015 Palanisamy, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.