Journal of
eISSN: 2373-4310


Research Article Volume 5 Issue 3
1Christian Doppler Laboratory for Innovative Bran Biorefinery, Austria
2Department of Food Science and Technology, University of Natural Resources and Life Sciences, Austria
3GoodMills Polska Grodzisk Wlkp. Grodzisk Wlkp, Poland
Correspondence: Silvia Apprich, Department of Food Science and Technology, University of Natural Resources and Life Sciences, Muthgasse 18, Vienna, Austria,, Tel +43-147-654 6635, Fax +43 1 47654 6629
Received: November 09, 2016 | Published: November 21, 2016
Citation: Prückler M, Apprich S, Gutsche M, et al. Influence of enzyme treated wheat bran on the dough rheology and texture characteristics of high fibre wafers. J Nutr Health Food Eng. 2016;5(3):613-621. DOI: 10.15406/jnhfe.2016.05.00171
To date, wheat bran has been mainly used in animal feeding. However, the bran fraction contains several valuable compounds with the potential to improve the nutritional quality of food and confectionery. Unfortunately, supplementation of wafers with wheat bran causes high viscosity of the resulting dough which impedes the use of standard production settings for wafer manufacture. The aim of this study was to achieve a viscosity reduction in bran containing wafer dough (20% wheat bran supplementation) through the application of carbohydrate degrading enzymes. Synergistic effects of a xylanase preparation combined with a recombinant arabinosidase reduced the dough viscosity to the level of bran-free dough and improved the dough transport through nozzles, the drop separation and the out-spreading on the wafer hot plates. The maximum and total breaking force, maximum breaking sound and number of acoustic peaks of the final wafers, characterizing the crispiness, were affected only to some minor extent by addition of pretreated bran.
Keywords: wheat bran, enzyme treatment, wafer dough, viscosity, textural parameters
AACC, american association of cereal chemists; AX, arabinoxylan; AXHd3, arabinoxylan arabinofuranohydrolase d3 specificity; DF, dietary fiber; ETB, enzymatically treated bran; FA, ferulic acid; FAE, ferulic acid esterase; ROV, run out viscosity; RVA, rapid visco analyzer; WE-AX, water extractable arabinoxylan; WU-AX, water insoluble arabinoxylan
Wheat bran accounts for about 15% of the grain weight1 and are an abundantly arising by-product of the milling industry. It contains about 13-18% protein, 3.5% fat and 56% of carbohydrates; 70-90% of these are dietary fiber (DF).2 Traditionally, bran is mainly used for animal feed purposes, but considering its composition, it could also serve as a source of dietary fiber for human consumption. Bakery products are an evident choice to integrate wheat bran into the food chain; and the use of reconstituted whole-grain flour or fiber fortified flour may contribute to improve public health. However, adding wheat bran to baked goods gives rise to difficulties such as sensory drawbacks, decreased baking volume and darker color.3 In addition, rheological difficulties result from the addition of bran to wafer dough, mainly due to its high water binding capacity which leads to high viscosity of dough’s. Such bran-containing dough is difficult to process using the standard wafer production equipment as the dough cannot be conveyed through the nozzles. This problem further impedes clean drop separation, whereby the dough does not spread out evenly on the wafer hot plates.4
In principle, the geometry of the nozzle could be changed; however, changing equipment is time consuming due to required cooling and heating times. Reduction of dough viscosity could also be achieved by increasing the water content. However, water vapour acts as raising agent, and more moisture consequently softens the wafers.5 As this disadvantageous phenomenon of dough’s containing bran is caused by typical cell wall components of the bran particles, mainly arabinoxylans (AX) and also by interactions between gluten and AX,6 a too viscous batter with strong water-binding capacity is generated. Additionally, bran particles act as charged filler in the gluten network and increase viscosity.7 AX consists of long xylose backbones with arabinose side chains. The latter can be further substituted with ferulic acid that forms intra molecular diferulic acid bridges through oxidative cross linking. Water retention of cell wall components as well as gluten aggregation can be reduced via enzymatic treatment of the bran. Enzymes of interest are (endo-) xylanases, cellulases, arabinofuranosidases or ferulic acid (FA) esterases as well as combinations thereof. Endoxylanase activity is found endogenously in wheat, being responsible for hydrolyzing the endosperm cell walls during germination.8 The addition of xylanases to dough causes an extensive degradation of water unsoluble arabinoxylans (WU-AX) and water extractable arabinoxylans (WE-AX) to oligomers, leading to a decrease in water absorption, and thus, to a decline in dough viscosity.9 The action of cellulases is analogous to xylanases, however, the effect in wheat bran is minor due to the presence of lignin and the low amount of cellulose compared to AX. Increased hydrolysis efficiency can be reached by combining the effects of xylanases with enzymes specifically targeting the AX side chains. A synergistic effect of xylanase together with ferulic acid esterase on aleuron cells was attested by Hell et al.,10 Likewise, Uraji et al.,11 reported that arabinofuranosidase, ferulic acid (FA) esterase and acetyl xylan esterase show synergistic effects in combination with xylanases, supporting AX degradation and increasing the effectiveness of the FA release.
Taking into account the above mentioned strategies, the aim of this study was to investigate the impact of enzymatic bran pre-treatment on the rheology of wafer dough fortified with wheat bran, based on run out viscosimetry (ROV) and on measurements with the Rapid Visco Analyzer (RVA). Furthermore, a preliminary evaluation on the effects on color and texture of the resulting wafers was performed, since wafer texture is significantly affected by moisture content.12 Last but not least, it should not be forgotten that increasing the dietary fiber content of wafers could change their unfavorable reputation from being a rather unhealthy, high caloric sweet to one that also could qualify as a snack with improved nutritional quality.
Raw materials
Fine (F500) & extra fine (F180) wheat bran (food grade, from mechanically cleaned and peeled wheat) and wheat flour (W700/DIN450) were provided by Good Mills Group, Schwechat, Austria. The fine fraction was taken from the 500µm sieve deck with 80% of the particles being smaller than 250µm, the extra fine fraction from the 180µm sieve deck of passage 9 with 80% of the particles being smaller than 125µm. In this study different types of commercially available xylanases, cellulases, FA esterases, glucanases, proteases and combinations thereof were tested compare Table 1. AXHm3 arabinofuranosidase from Lactobacillus brevis (XynB2) was produced recombinant as recently described Michlmayr et al.13 Applying the same procedures as reported for AXHm3, AXHd3 from Bifidobacterium adolescentis14 was expressed in E. coli using the pET21a expression system and purified to homogeneity.
Name |
Company |
Activity |
Lot Nr. |
Tegazym X100P |
Tegaferm |
Xylanase |
Y555 |
Tegazym CEL-P |
Tegaferm |
Cellulase |
Y554 |
Sternzym |
Sternenzym |
Ferulic acid esterase |
FSR2201 |
Pentopan 500 BG |
Novozymes |
Xylanase |
CF102247 |
AMG1100BG |
Novozymes |
Glucoamylase |
AM104009 |
Ultraflo L |
Novozymes |
b-Glucanase, cellulase, Xylanase |
CAN2102 |
Neutrase 0.8L |
Novozymes |
Protease (neutral) |
PWN10073 |
AXHd3 |
- |
Arabinofuranosidase |
- |
AXHm3 |
- |
Arabinofuranosidase |
- |
Table 1 Enzymes used in this study
Determination of basic nutritive parameters
All bran and flour samples were analysed for dry matter and ash content, crude fat and protein content, starch and -glucan, soluble and insoluble dietary fiber. The results are given in Table 2. The dry matter content was determined according to AACC 44-15.02 weighing 2g of sample. All further results were related to dry matter content. For the ash content (according to AACC 08-01.01) also 2g aliquots were used. Crude fat and crude protein were measured according to AACC 30-25.01 and AACC 46-12.01. Starch, b-glucan, soluble and insoluble DF contents were determined using the Megazyme test kits total starch (K-TSTA), b-glucan (K-BGLU) and total dietary fibre (K-TDFR), respectively.
Material |
F500 |
F180 |
W700 / Strong Gluten flour |
Dry matter |
91.6±0.8 |
93.6±0.8 |
87.3±0.7 |
Ash |
5.8±0.1 |
3.3±0.0 |
0.8±0.0 |
Crude fat |
5.7±0.1 |
4.9±0.0 |
1.3±0.1 |
Crude protein |
15.5±0.1 |
15.0±0.1 |
13.9±0.1 |
Starch |
17.6±0.2 |
35.1±0.8 |
65.1±0.6 |
b - Glucan |
2.7±0.1 |
1.9±0.0 |
0.3±0.0 |
Soluble dietary fibre |
3.4±0.1 |
5.1±0.1 |
1.1±0.0 |
Insoluble dietary fibre |
40.5±0.4 |
29.8±0.3 |
3.4±0.1 |
Table 2 Basic compositional details of food grade wheat bran and wheat flour W700 in [%]. All values are means of triplicate analyses (n=3) and refer to dry matter
Preparation of doughs with and without wheat bran
For each measurement the weighed amount of enzyme (0.1% of bran weight) was mixed with 620.5 g of tap water in a mixing bowl until homogeneity; 100g of bran were added subsequently resulting in 87% (w/w) moisture. The water addition was adjusted to different materials to result in 61% (w/w) dough moisture. After homogenization for 10min the mixture was covered with aluminum foil and placed in an oven at 40°C. After 4h, two 1mL aliquots of the water phase were heated to 80°C in a water bath to inactivate enzymes and microorganisms and to precipitate protein, then centrifuged at 20,800g for 3min to obtain a clear sample and frozen at -30°C for sugar analysis. 400g of flour were added to the remaining suspension, resulting in 61%(w/w) moisture, and mixed at 125rpm for 120 s. After mixing, the dough temperature was 30±1°C. The control dough was prepared analogously, containing no bran but 500g flour and 615g of tap water instead (resulting in 61% (w/w) moisture). This recipe was based on the one used by Gluszczynski, Hanneforth, Lindauer & Brümmer, (2001). Due to the previous bran pre-treatment and different water holding capacities of the flour-bran mix, adaptions were made in temperature (+20%), mixing velocity (+100%) and in water addition (+0.8%).
Determination of run out viscosity (ROV)
ROV measurement was carried out according to Hanneforth and others (1997). The prepared wafer dough was filled into an outflow viscometer above the top measuring line. Time recording was started when the dough reached the top measuring line and stopped when the dough reached the bottom measuring line. The volume between top and bottom measuring line was 200mL, and the brass nozzle (70mm long and 8mm inner diameter) was analogous to the production equipment. The dough was covered, stored at room temperature and the test was repeated after 30min to evaluate the time dependence of viscosity.
Determination of viscosity by rapid visco analyzer (RVA)
Three aliquots of 20g of each of the wafer dough prepared as described in 2.2 were transferred to aluminum cans and placed into the RVA 4500 (Perten Instruments Pty. Ltd., Warrie wood, Australia). The mixing conditions were 125rpm for 120 s at 25°C. Subsequently, the viscosity was measured. The dough was covered, stored at room temperature, and the test was repeated after 30min to evaluate the time dependence.
Baking trials, color measurement and texture determination
Ten grams of the dough were poured on the hot plate and baked in the Glutork 2020 dryer (Perten Instruments, Huddinge, and Sweden) for 4minutes at 150°C. The wafer was taken out and allowed to cool to room temperature. Reflectance colorimetric measurement (L*a*b, viewing geometry: d/8°) was done by Dr. Lange Spectrocolor LMG183 (Dr. Bruno Lange GmbH & Co.KG, Düsseldorf, Germany) in triplicates. The diameter of the wafer sheet was measured at 5 points (data not shown) and then it was cut into shape (48x100mm) for texture analysis, which was done by recording acoustic breaking events, breaking force and distance with a TA.XT plus Texture Analyser (SN: 11455, Stable Microsystems Ltd., Surrey, UK) using a Craft Knife Adapter (model A/CKB, 50mm) and a fixture (model HDP/90). The pretest speed, test speed, and post-test speed were 1.0mm/s, 5.0mm/s and 10.0mm/s, respectively. The test distance was 50.0mm and contact force was 5g. The data acquisition rate rate was 250 PPS. The microphone (Brüel & Kjaer Type 4188-A-021, serial no. 02525762) was placed in 30mm distance to the sample. Wafers were stored at 20°C and at 50% relative humidity for 4weeks and measured again.
Determination of free sugars
The free sugars (glucose, xylose, arabinose and galactose) in the water phase after bran treatment were measured according to Sluiter & Sluiter, 2011. The sugars were separated using a Rezex RPM-Monosaccharide column equipped with a guard-column (Phenomenex LTD, Aschaffenburg, Germany).
Statistical methods
RVA viscosity and color were measured in triplicate; breaking tests were done in quintuplicate and analyzed using stat graphics Centurion XVI (version 16.0.09, Stat Point Technologies, Inc., Warrenton, USA). ROV measurements lower than 10min were done in duplicate, higher than 10min once to comply with the 30min interval for the 2nd measurement. Initial values of wafer dough containing no bran and untreated bran were measured with n=3 for ROV and n=20 for RVA. A 0.05 alpha level was used as a significance level for all statistical tests.
Analysis of raw materials
The nutritive parameters of the used materials are shown in Table 2. The two bran fractions differed especially in terms of starch and dietary fiber (DF) contents.
Viscosity measurements of reference, control and sample doughs
ROV measurement is closer to the production process, however high viscosity doughs result in long measuring time and high variance. The RVA, usually used for pasting behavior, is used for an expeditiously dough viscosity measurement at constant temperature. Viscosity measurements were repeated after 30min to evaluate the time dependence. The findings of the 2nd measurement are given in brackets. Results are shown in Table 3.
Bran Pre-Treatment |
Viscosity [Pa.S] |
|
Measurement time |
0 min |
30 min |
no bran (´reference´) |
1.8±0.1a |
1.5±0.1a |
F500 moistened without enzymatic treatment (´control´) |
6.2±0.4c |
5.5±0.4c |
F180 moistened without enzymatic treatment |
3.7±0.3b |
3.4±0.3b |
F500 hydrothermally treated (100°C; 30 min) |
11.3±0.6d |
10.0±0.1d |
F500 enzyme treated (X100P & CEL-P; 0.2% each) |
3.8±0.5b |
2.9±0.3b |
F500 hydrothermal & enzyme treated (X100P & CEL-P; 0.2% each) |
3.8±0.4b |
2.8±0.3b |
Table 3 Viscosity (Rapid ViscoAnalyzer) of wafer doughs prepared with various pre-treated brans (pre-moistening, hydrothermal pre-treatment alone and combined with enzymatic treatment), n=3, Values labelledwiththe same superscriptletterare not different at p < 0.05 in Fishers LSD multiple comparisons of means
The dough used for reference purposes (W700 wafer dough) consisted of pure flour with a water content between 61.0% and 61.1% instead of bran and had a mean viscosity of 1.8Pa.s (1.5Pa.s) (n=20). In the ROV method, W700 wafer dough had an outflow time of 9min (12min) (n=3). Wafer dough containing 20% bran F500 (bran was milled and soaked in water, but not enzymatically modified, referring as ´control´) had a viscosity of 6.2Pa.s (5.5Pa.s). Unfortunately, it had a high ROV of about 20min (40min), presumably caused by blockage of the nozzle, as judged from inhomogeneous flow. By applying the same procedure to bran F180, the viscosity was 3.7Pa.s (3.4Pa.s) and ROV 18min (40min). Despite the different composition of the two bran fractions and the high differences in viscosity (Table 3), there was no apparent effect on ROV between doughs containing bran F500 and bran F180. This could be explained by the influence of gluten fibrils on ROV (blockage of the nozzle), but not on RVA measurement (Auger, Morel, Lefebvre, Dewilde & Redl, 2008). Thus, it shows that the use of finer bran fractions does not improve the workability. To evaluate the effect of a hydrothermal step in order to inactivate endogenous enzymes and microbial contaminations, 100g of bran F500 were cooked for 30min at 100°C. After cooling to 40°C- and optional enzyme treatment with a mixture of X100P and CEL-P, 0.2% (w/v) each, based on results from pre-trials, - the dough was prepared and viscosity determined. Cooking nearly doubled the viscosity to 11.3Pa.s (10.0Pa.s), most likely due to starch gelatinization and inactivation of endogenous enzymes. However, there was no difference in measured viscosity after enzyme treatment with and without previous hydrothermal treatment. Enzyme treatment reduced viscosity in both dough’s to 3.8Pa.s.
The effect of increasing moisture and enzyme concentration on dough viscosity was evaluated in the bran F500 dough (Figure 1A) (Figure 1B). In the range of moisture investigated (60.5 to 68%), the decrease in viscosity is about 0.36 Pa.s per one percent increase of moisture. The evaluated range of enzyme mix (X100P and CEL-P 1:1) was in the level of 0 to 0.4%. The decrease in viscosity was linear in the evaluated range 8.2Pa.s per percent of added enzyme mix, Figure 1B.
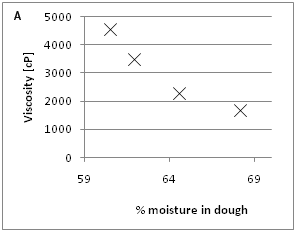
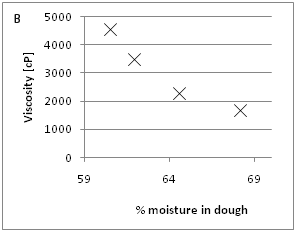
Figure 1 a: Effect of moisture levels on the viscosity of resulting wafer doughs containing 20% of bran F500 ranging from 60.5 to 68% moisture (n=3).
b: Effect of various enzyme concentrations (0 to 0.4%) on the viscosity of resulting wafer doughs containing 20 % bran F500 (n=3).
Xylanase and cellulose show analogous activities. In general, the addition of these enzymes causes the hydrolysis of cell wall carbohydrates. Endoxylanase decreases the degree of polymerization in AX - and after prolonged reaction time soluble AX polymers are further degraded to smaller oligomers.15 Thus, water absorption and viscosity are decreased. However, even if an extensive degradation of AX to oligomers leads to a decrease in water absorption and viscosity reduction, growing gluten aggregates may again increase the dough viscosity.16 Although the reaction mechanism of cellulose is similar to that of xylanase, due to the presence of lignin the effect in bran isminor compared to flour.17
Effects of commercially available enzymes in combination with X100P and CEL-P
The above described results showed that the preparations X100P and CEL-P are generally capable of reducing the viscosity of wheat bran containing doughs. Improved performance could be reached by combining the xylanase/cellulase mixture with enzymes acting on other components present in the dough. To assess this, four different enzymes (Table 1) were added together with 0.1% X100P and 0.1% CEL-P and compared to moistened bran in order to consider possible synergistic effects. The results are shown in Figure 2A & 2B. The effectiveness of combining a xylanase together with an esterase on aleuron cells was attested by Hell and co-workers.10 Therefore, it was of interest for this study to evaluate if and how the addition of ferulic acid esterase would influence dough viscosity.
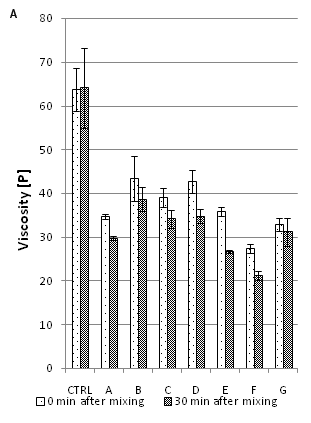
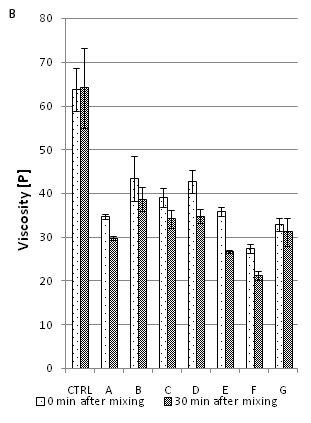
Figure 2 Effect of enzyme treatment of bran F500 on resulting wafer dough viscosity (a) and ROV (b); CTRL: moistened bran F500; A: 0.1% X100P; B: 0.1% CEL-P; C: 0.1% X100P & 0.1% CEL-P; D: C & 0.1% AMG1100BG; E: C & 0.1% Ultraflo L; F: C & 0.1% Neutrase; G: C &Sternzym, Values labelledwiththe same superscriptletterare not different at p < 0.05 in Fishers LSD multiple comparisons of means.
Compared to solely X100P and CEL-P treated bran, the additional application of amylase (D, AMG1100BG) failed to reduce viscosity or ROV levels. FA-esterase (G, Sternenzym) and cellulase alone (B, CEL-P) reduced viscosity, but increased ROV. These enzymes have a limited effect on AX solubilization, a fact that was previously mentioned by Figueroa-Espinoza, et al.,18 and Petit-Benvegnen, et al.,19 b-Glucanase (E, Ultraflo L) and protease (F; Neutrase 0.8L) could reduce dough viscosity by 8%(22%) and 30%(38%), respectively. Similar effects were detected regarding ROV – a reduction by 1%(14%) for Ultraflo L and 24%(85%) for Neutrase 0.8L. Nevertheless, especially the protease exerted full effectiveness only after flour addition, presumably due to reactivity with protein primarily available in the flour. Therefore, the time dependent effect of 0.1% Neutrase 0.8L on viscosity levels was evaluated. The viscosity of the bran pre-treated with protease directly after mixing with flour was not significantly different to that of the untreated wheat bran, however, after 30min, the viscosity was reduced by 27%, and after 60min by 46%. This viscosity reduction initiated by flour addition shows that the protease only affects the flour proportion of the dough. Thus, further experiments were directed towards the effect of enzymes acting on AX in bran. Most effective, directly after mixing, was 0.1% X100P alone, followed by the combination of X100P with CEL-P (0.1% each) (Figure 2A) (Figure 2B).
Synergistic effects of arabinoxylan degrading enzymes
Apart from enzymatic hydrolysis of the xylan backbone or of other components of bran, the arabinose residues can also be targeted in order to reduce the viscosity enhancing effect induced by AX. Uraji et al.,11 reported that arabinofuranosidase, ferulic acid (FA) and acetyl xylan esterases exert synergistic effects in combination with xylanases, supporting AX degradation and increasing the effectiveness of the FA release. Arabinofuranosidases cleave arabinose residues from the xylan backbone, FA esterase hydrolyses the bond between arabinose residue and FA, and acetyl xylan esterase supports the degradation of AX. According to van den Broek et al.,14 arabinoxylan arabinofuranohydrolase-D3 (AXHd3) pre-treatment improves the extent of a subsequent b-xylanase hydrolysis in soluble wheat AX.
Two arabinosidases, AXHd3 and AXHm3, were tested for their synergistic effect with xylanase, 0.1% X100P or 0.1% Pentopan 500BG upon bran pre-treatment. For reference purpose combinations of all four enzymes ('All') and all four with FA esterase ('All' + FAE) were added. The results are shown in Figure 3A & 3B. X100P in combination with AXHm3 resulted in a 16% (23%) reduction of viscosity in RVA measurement compared to X100P alone and reached a viscosity similar to the reference dough with flour W700. These two enzymes were also identified based on a multiple regression after Box-Cox-Transformation (power=-0.316 and shift=0) and in a correlation analysis to demonstrate any influence on viscosity reduction. Addition of AXHd3 to X100P caused no significant reduction of viscosity in RVA measurement compared to X100P alone. Pentopan 500BG alone exerted an effect on the viscosity showing an increase by 15% compared to the control dough (pre-moistened wheat bran F500) but showed less effect than X100P, even in combination with arabinosidase. The addition of FAE to the mix of all enzymes was able to reduce the viscosity (further) by 16%. Nevertheless, these controls had higher ROV than X100P alone. In general, the ROV measurement yielded a similar trend as the RVA measurements. Only X100P, together with AXHm3 treatment, resulted in lower ROV values compared to X100P alone. All other combinations had ROV times between 13 and 18min (17 to 29min). The monosaccharide contents in the supernatants after enzyme treatment shown in Supplemental Table S1 generally correlate with the observed activity of the different enzymes.
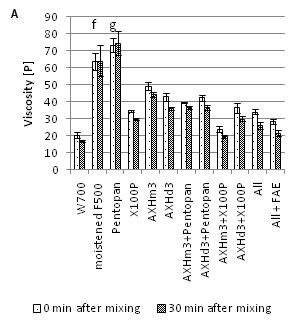
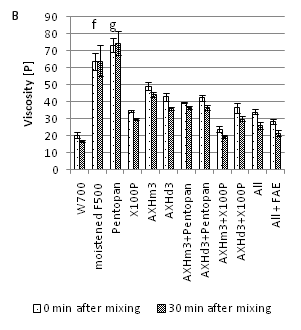
Figure 3 Effect of bran treatment with various AX degrading enzymes on wafer dough viscosity (a) and corresponding run out viscosity (b), Samples labelledwiththe same superscriptletterare not different at p < 0.05 in Fishers LSD multiple comparisons of means.
Impact of enzyme treatment on wafer color and texture
In general, wafers containing enzymatically pretreated bran were of darker color than those containing untreated bran (Supplemental Table S2). Hereby, a multiple regression after Box Cox Transformation showed a significant impact of free glucose and galactose content (Table S1) on wafer darkness, which is due to Maillard reactions. There was no significant effect on the a* axis of the L*a*b*-system, however, enzyme-based hydrolysis seemed to shift the wafer color towards magenta on the b* axis data shown in the supplement Table S2.
Interesting results were found for the crispiness of the resulting wafers (Table 4), as characterized via ´maximum´ and ´total breaking force´, ´maximum breaking sound´ and ‘number of acoustic peaks‘. Bran is known to be very hygroscopic, a property that could shorten the shelf life of wafers due to softening of the wafer by absorbing humidity from the surroundings. However, there was no significant difference observed between wafers with and without bran incorporation from the ´maximum breaking force´ (21-34 N) and ´maximum breaking sound (84-88 dB)´measurements, neither for freshly baked nor for 4weeks old wafers stored at 20°C and at 50% relative humidity. Significant differences or even trends derived from different enzyme combinations were found for ´total breaking force´ [N mm] and ´number of acoustic peaks´. Regarding the ´total breaking force´ within the fresh products highest values were measured for pure flour wafers (15 N mm), followed by wafers containing 20% bran treated with one of the xylanases or all enzymes (9 to 11 N mm) and wafers containing 20% bran treated with one of the arabinosidases (5 to 8 N mm). The lowest breaking force levels were registered with wafers containing 20 % native, moistened bran (5 N mm). The ´total breaking force´ of aged wafers produced with pre-treated bran was higher than that of the fresh counterparts. This effect was most pronounced in the wafers produced with Pentopan 500BG and AXHm3. This implies that wafers containing enzymatically treated bran (ETB) seem to become harder during the storage period in contrast to the reference wafers without bran. However, this effect was less pronounced in wafers produced with untreated and excessively treated bran, thus further investigation in this regard is warranted. Regarding the number of acoustic peaks upon breaking reference wafers (4.8 acoustic peaks) were lowest, while control wafers were highest (17.2 acoustic peaks). Wafers with ETB ranged from 6 to 17 peaks, exhibiting higher peak numbers when wafers were older.20‒26
Enzymatic |
Age [W] |
Max. Force [N] |
Total Breaking Force [N mm] |
Max. Breaking Sound [dB] |
Number of Acoustic Peaks |
|
Reference (W700) |
fresh |
27±6c |
15±4d |
85±1a |
5±1a |
|
4 weeks |
12±3a |
5±1a |
88±1a |
12±2c |
||
Control (moistened |
fresh |
23±3b |
5±2a |
86±1a |
17±3e |
|
F500) |
4 weeks |
22±5b |
6±2a |
87±2a |
14±4d |
|
Pentopan 500BG |
fresh |
26±5c |
11±3c,d |
87±2a |
12±3c |
|
4 weeks |
30±11d |
14±5d |
87±1a |
13±2c |
||
X100P |
fresh |
24±4b |
9±4b,c |
86±1a |
12±4c |
|
4 weeks |
34±11d |
17±6e |
87±2a |
15±2d |
||
AXHm3 |
fresh |
33±4d |
8±1a,b,c |
84±1a |
9±1b |
|
4 weeks |
22±4b |
11±2c,d |
88±1a |
17±6e |
||
AXHd3 |
fresh |
21±6b |
6±1a |
86±2a |
8±2a,b |
|
4 weeks |
27±7c |
15±5d |
87±1a |
16±2d |
||
AXHm3 +Pentopan 500BG |
fresh |
22±2b |
5±1a |
85±2a |
8±1a,b |
|
4 weeks |
33±9d |
19±8e |
87±2a |
17±3e |
||
AXHd3 +Pentopan 500BG |
fresh |
28±5c |
7±1a,b |
84±2a |
7±1a,b |
|
4 weeks |
23±7b |
13±3d |
86±1a |
17±4e |
||
AXHm3 + X100P |
fresh |
26±6c |
7±2a,b |
85±1a |
10±1b,c |
|
4 weeks |
23±5b |
9±2b,c |
87±2a |
13±2c |
||
AXHd3 + X100P |
fresh |
22±8b |
6±1a |
87±2a |
9±3b |
|
4 weeks |
32±5d |
14±3d |
87±1a |
13±3c |
||
all |
fresh |
25±3b |
9±1b,c |
85±1a |
10±1b,c |
|
4 weeks |
24±3b |
11±1c,d |
87±1a |
14±3d |
||
all+Sternzym |
fresh |
27±5c |
10±2c,d |
87±1a |
9±2b |
|
4 weeks |
22±3b |
10±2c,d |
87±1a |
15±2d |
||
Table 4 Effect of enzymatic pre-treatment of bran on textural attributes of corresponding wafers under fresh and stored conditions (n=5), Values labelledwiththe same superscriptletterare not different at p < 0.05 in Fishers LSD multiple comparisons of means
Our experiments showed that the high viscosity of wafer doughs containing wheat bran can effectively be reduced through technologically simple enzymatic treatment. However, application of enzymes at doses typical for bakery usage generally showed minor effectiveness. High dosage and long process times usually are economically unfeasible and increase the risk of microbiological spoilage. The most promising effects were obtained with bran pre-treated with the xylanase preparation X100P in combination with arabinosidase AXHm3. Dough viscosity values similar to those of wafer dough without wheat bran addition could be achieved. Upon bran pre-treatment xylanase and arabinosidase exert their cleavage potential towards the hemicellulose. Arabinosidase hydrolyses the terminal non-reducing alpha-L-arabinofuranosidases residues resulting in less branching sites in the AX. Xylanase then degrades the linear xylan backbone. Through combined application, both the water holding capacity of AX and the dough viscosity were significantly reduced. Hence, the transport flow of the dough through the nozzles, the drop separation and the spreading out capacity on the wafer hot plates were improved. The influence of bran addition and bran pre-treatment on maximum breaking force and maximum breaking sound of the produced wafers was not significant. We observed that, as in the reference wafers without bran, in bran containing wafer samples the number of acoustic peaks is increasing over the storage period. Regarding hardness as characterized by total breaking force the staling effect is different between wafers with and without bran addition. Wafers without bran tend to decrease in total breaking force during storage due to absorbed moisture. However, wafers containing bran increase in total breaking force over the storage period. We therefore hypothesize that moisture is inertly stored in bran fibers instead of softening the starch-protein matrix. Clearly, a different approach using a larger sample set will be required to further prove this assumption.
From the technological point of view the application of wheat bran to wafers is a challenging task although viscosity reduction by enzymatic pre-treatment seems to be useful as the textural impact is not as large as expected. Nevertheless, from the economic perspective, the application of enzymes at a larger scale may not be easy due to the high enzyme doses required and the lack of suitable food-grade enzymes.
GoodMills Group GmbH, Austria and the Christian Doppler Forschungsgesellschaft, Austria are acknowledged for their financial support. We also thank Sternenzym for providing the FAE Sternzym.
Author declares that there is no conflict of interest.

©2016 Prückler, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.