Journal of
eISSN: 2373-4310


Review Article Volume 3 Issue 1
Department of Health, Baylor University, USA
Correspondence: Darryn S Willoughby, Department of Health, Human Performance and Recreation, Exercise and Biochemical Nutrition Laboratory, Baylor University, Waco, TX 76798, USA, Tel 254-710-3504
Received: November 08, 2015 | Published: December 3, 2015
Citation: Marroquín FEM, Willoughby DS. Factors regulating uncoupling protein-3 (ucp-3) expression in skeletal muscle. J Nutr Health Food Eng. 2015;3(1):285-291. DOI: 10.15406/jnhfe.2015.03.00101
Nutrition and physical activity are environmental factors that play a vital role in health maintenance. Both are part of the first line of obesity treatment. The physiological effects of physical activity have been known for decades; however, it was not until recently that the biochemical and molecular effects started to receive special attention. Uncoupling proteins (UCP) are mitochondrial proteins that produce heat by dissipating the electrochemical potential located in the inter-membrane space. UCP have shown to protect against reactive oxygen species, lipid peroxidation, free fatty acid accumulation, and insulin resistance, at the same time of increasing metabolic rate. Three isoforms have been detected in humans, UCP-1, UCP-2 and UCP-3, with UCP-3 being the predominant isoform expressed in skeletal muscle. Two transcription factors that have been proposed as important regulators of UCP expression activation are peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC1-α) and peroxisome proliferator-activated receptor alpha (PPARα). Both transcription factors play a central role in the response to external stressors, like fasting and exercise. β-aminoisobutyric acid (BAIBA), a small molecule produced by PGC-1α, is a myokine that has recently been shown to produce browning of white adipose tissue and increased β-oxidation in hepatocytes by acting through PPARα. In vitro, BAIBA treatment has shown to increase mitochondrial respiration and the expression of UCP. The main objective of the present review is to provide a discussion with relevant information regarding various factors that regulate UCP-3 expression in skeletal muscle.
Keywords: uncoupling protein, skeletal muscle, aminoisobutyric acid, peroxisome proliferator-activated receptors
UCP, uncoupling proteins; PGC1-α, peroxisome proliferator-activated receptor gamma co-activator 1 alpha; PPARα, peroxisome proliferator-activated receptor alpha; BAIBA, β-aminoisobutyric acid; ROS, reactive oxygen species; FFA, free fatty acids; ETC, electron transport chain; ATP, adenosine triphosphate; O2, oxygen; EPOC, excess post-exercise oxygen consumption; FEE, factorial energy expenditure; HIRT, high-intensity interval resistance training; CPT-1, carnitine palmitoyltransferase-1; PPARS, peroxisome proliferator-activated receptors; CIDEA, cell death-inducing dffa-like effector A
According to the World Health Organization,1 obesity rates have nearly doubled since 1980, increasing the prevalence of chronic comorbidities and causing around 2.8million deaths per year.2,3 Among other important factors, obesity development is believed to be mainly generated by energy imbalances, either increased energy consumption, decreased energy expenditure, or both.4‒6 Nutrition and physical activity are environmental factors that play a vital role in health maintenance. Both are part of the first line of obesity treatment. According to Speakman and Selman,7 in order to achieve the energy expenditure of our primitive ancestors, it would be necessary for humans to engage in 90min of intense daily physical activity. Moreover, it has been shown that high levels of physical activity decreases mortality rates, an effect that is further amplified when the exercise is performed at high intensity.8 One of the genes affected by exercise is the gene controlling uncoupling proteins (UCP). UCPs are mitochondrial proteins that dissipate the inter-membrane electrochemical potential as heat, by transporting protons back into the mitochondrial matrix.9 UCPs are classified in three isoforms, UCP-1, UCP-2 and UCP-3. From the three isoforms, UCP-3 is the predominant isoform expressed in skeletal muscle.9,10 This mitochondrial protein has shown to protect against reactive oxygen species (ROS), lipid peroxidation, FFA accumulation in skeletal muscle, and insulin resistance, at the same time of increasing metabolic rate in skeletal muscle.9,11 Two of the transcription factors that have been proposed as important regulators of UCP expression activation are PGC-1alpha and PPARgamma. However, evidence is lacking.12,13 Both transcription factors play a central role in the response to external stressors, like fasting and exercise.14,15
Exercising during fasting conditions induces UCP-3 mRNA expression in skeletal muscle, which is decreased by glucose consumption before and during the exercise session. The explanation to the mentioned effect was a possible involvement of free fatty acids (FFA) controlling UCP-3 expression. Conflicting results have been found16‒18 indicating that the consumption of diets with different macronutrient composition seem to have an opposite effect on the exercise-induced UCP-3 mRNA expression in skeletal muscle. While a high-protein and a high-fat diet have shown to increase UCP-3 expression by themselves, a high-carbohydrate diet has the total opposite effect.19‒21 Until now studies assessing the effect of exercise on UCP-3 expression in skeletal muscle have used only glucose as a sole energy source. Additionally, the glucose provided was consumed both before and during the exercise session.14,16 Most individuals typically ingest multi-nutrient meals prior to exercise, ingesting only carbohydrates as the sole energy source during an exercise session might not represent a typical, practical scenario. Moreover, most of the times physically active people will find themselves consuming food before or after the exercise session but not during. The development of projects assessing the consumption of a multi-macronutrient diet just before the exercise should be used in order to assess UCP-3 expression in a more realistic scenario.
The United States of America has one of the highest obesity rates in the world. Approximately 34.4% of the American population is considered to fall within the obesity range.22,23 According to the results of the analysis performed by Preston and Stokes,23 no other country is estimated to gain as much benefit from the elimination of obesity as the United States. A proper program of physical activity and healthy nutrition are known as the first line of treatment against obesity. The actual recommendation of physical activity is 2.5h of moderate aerobic exercise per week with muscle-strengthening activities at least two days per week.24 According to Samitz and colleagues,8 the mortality reduction for this amount of physical activity (150 to 300min per week of moderate to vigorous intensity) would be about 14 to 26%. During exercise, the increased metabolic demands produce a higher rate in the respiratory chain and the oxidative phosphorylation machinery to improve adenosine triphosphate (ATP) production. The mitochondrial electron transport chain (ETC) moves electrons from an electron donor (e.g. reduced nicotinamide adenine dinucleotide or reduced flavin adenine dinucleotide, NADH and FADH2 respectively) to a terminal electron acceptor (Oxygen, O2), while simultaneously pumping protons from the matrix to the intermembrane space. These coupled reactions produce a gradient potential that is further utilized to produce ATP and H20.25 The O2 consumption by the mitochondria (in order to form H20 molecules) is the reason why it is commonly stated that the real respiratory machinery is not located in the lungs but within the mitochondria itself. This would indicate that the higher the ATP demand, the greater the necessity of O2, which would consequently increase the breathing frequency during physical activity. Additionally, once the exercise session is finished, the increased O2 consumption continues during the post-exercise phase. In the past, it was believed that this excess in O2 consumption was caused by the glycogenesis of the lactate produced during physical activity. However, it seems that there is no such relationship between lactate metabolism and O2 consumption after exercise. According to Gaesser & Brooks,26 less than 20% of the lactate is re-converted to glycogen while around 55 to 70% is oxidized, which would indicate that oxidation is the primary post-exercise pathway of exercise-produced lactate. Moreover, it has been shown that the rest of the O2 consumed after exercise is explained by numerous factors such as: recovery from decreased muscle glycogen levels, creatine-phosphate restoration, increased sympathetic tone and circulation of stress hormones.26,27 In fact, Gaesser & Brooks26 proposed to change the terms “lactacid”, “alactacid” and “O2 debt” to “excess post-exercise oxygen consumption” (EPOC). An increased EPOC means that there is extra energy consumption above the resting metabolic rate after the cessation of the exercise session. According to “The Institute of Medicine,” when calculating the energy expenditure of physical activity, an additional 15% of the actual energy consumption during the exercise session should be added due to EPOC effects.28 This adjustment to the factorial energy expenditure (FEE) formula has been shown to be more effective than the estimation formula alone.29 It is worth noting that both aerobic and resistance exercise have been shown to increase energy expenditure, during both the exercise session and the post-exercise/recovery phase.27,30‒32
Using a metabolic chamber, Knab et al.,27 demonstrated a 37% increase in the energy expenditure during the post-exercise phase of a cycling session. In other words, burning around 519kcal per exercise session caused an increase of 190kcal in the recovery phase, generating a final energy expenditure of around 700kcal per day.27 A single bout of sprint exercise (225kcal) has shown to increase energy expenditure up to 2 to 3h post-exercise while a 45 min cycling session at 70% of VO2max caused a rise in the energy expenditure of up to 14h in the recovery phase.27,32 Intensity and duration of the exercise session are critical factors determining energy expenditure in the post-exercise period. As such, it can be suggested that a larger duration and a higher intensity of the exercise session will increase the post-exercise energy expenditure.
Resistance training may also prevent weight gain due to its long-term effects in energy expenditure. Kirk et al.,30 showed a significant increase in resting metabolic rate (120kcal) after a training period of 6months in a group of sedentary, overweight, young adults. These effects occurred even when the volume of the training program was relatively low at 11min per session with a frequency of three times per week.30 Importantly, the increase in metabolic rate was not taken in the post-exercise period but 72h after the last exercise session. This indicates that resistance training is able to increase the energy expenditure during resting conditions and not just during the recovery phase as it has been shown with endurance exercise,30,33 and could be the result of an increased fat-free mass within the resistance-training group.30 In resistance exercise, intensity also plays a crucial role. Paoli et al.,31 observed a 5% increase in the resting metabolic rate on a traditional resistance-training group after 22h of the last exercise session. Furthermore, they observed a 23% increase in the high-intensity interval resistance training (HIRT) group, even when the duration of the exercise sessions of the last group was shorter (62 versus 32min in the traditional and HIRT training groups, respectively). Likewise, it was demonstrated that when aerobic exercise is performed after 24 to 48h of unaccustomed resistance exercise, the energy expenditure increases 13% above a normal aerobic session. This could be the result of increased re-synthesis of damaged muscle fiber or increased muscle fibers recruitment to couple the metabolic demands.34 In summary, while aerobic exercise shows a significant increase in the post-exercise period, resistance exercise seems to have a slower but longer effect in energy expenditure. The combination of both resistance and endurance exercise might be the best strategy to increase metabolic rate in the short and long term setting.
Nutrition, before and after the workout, has been shown to modify the gene expression normally affected by exercise. It has been demonstrated that a low carbohydrate diet within 48h after a glycogen-depleting exercise can lead to a twofold increase in FFA concentrations along with increases in fatty acid translocase, UCP3, carnitine palmitoyltransferase-1 (CPT-1), β-hydroxyacyl-CoA dehydrogenase and hormone sensitive lipase.35 On the other hand, a high-carbohydrate diet in the post-exercise period can lead to a 300% increase in muscle glycogen content, along with increases in the transcription of the glucose transporter isoform 4 and glycogenin.35 These results could be used in one of two ways: 1) a high-carbohydrate diet in the post-exercise period could be useful for athletes and people engaged in sports in order to improve their performance, while, 2) a low carbohydrate diet in the post-exercise period could be used if an increase in energy expenditure is the desired result.
Adipose tissue is a major metabolic organ traditionally classified as either white (WAT) or brown (BAT) adipose tissue. WAT and BAT cell types are anatomically different and have antagonistic functions. On one side, WAT is characterized by single lipid spherical droplets with low mitochondria concentration and almost undetectable UCP-1 content. In contrast, BAT is characterized by multiple small vacuoles, abundant mitochondria, high UCP-1 content, and higher capillary and nerve supply than WAT, thus, producing the brown color. The UCP content in the internal membrane of the mitochondria can completely change its function. The mechanism through which mitochondria normally works is by letting the protons flow down the mitochondrial membrane through the ATP synthase. However, when the protons run back to the matrix through UCP (without ATP formation), the stored energy is dissipated as heat. While WAT main function is to store the energy consumed in excess, BAT function is to dissipate energy through heat production. It is interesting to note that, although they are derived from the same mesenchymal stem cell, WAT and BAT have different cell precursors. While WAT is originated from an adipogenic lineage, BAT is derived from the myogenic factor 5 lineage, the same precursor of myocytes. The transformation of this myogenic factor into BAT has been shown to be controlled by the growth factors: bone morphogenetic protein-7 and myostatin, as well as the transcriptional factors: CCAAT/enhancer binding protein β and PR domain containing 16 (PRDM16). Brown adipocytes and muscle cells are specialized in catabolism rather than anabolism, both cells are sympathetically innervated, with high amounts of mitochondria and possess adaptive thermogenesis.36 Until recently BAT was considered as irrelevant in adults. However, new research has shown the positive correlation between BAT and energy expenditure, as well as the negative correlation between BAT and body fat percentage. As such, new research is being done in this area with BAT becoming an attractive target for obesity treatment. Synthetic chemicals that activate the transcription factor PRDM16 or produce a similar action are also of potential interest as anti-obesity drugs.36
Recently, a new type of adipose tissue has been identified. Beige adipose tissue is also known as brite or brown-like adipose tissue. This new adipocyte type resembles WAT at basal conditions and is able to increase heat production and energy expenditure under certain stimulation. The adult human body has small depots of BAT, located mainly in the inter-capsular, axillary, paravertebral, and peri-renal areas. However, the discovery of this type of adipose tissue responsive to environmental changes that is able to resemble the function of BAT has captured the attention of scientists. Beige adipocytes are expressed within WAT, which is present throughout the body in two representative locations: visceral WAT and subcutaneous WAT. The former is located mainly around the organs providing padding, while the latter is found under the skin and its main purpose is to isolate the body from extreme temperatures. Besides sharing some similarities with WAT at basal conditions and with BAT under β-adrenergic stimulation or cold exposure, beige adipose tissue possesses its own gene expression pattern. The characteristic genes of BAT are: UCP-1, epithelial V-like antigen 1, pyruvate dehydrogenase kinase isozyme 4, early B-cell factor 3 and heat shock protein B7; while for WAT these genes are: adiponectin, resistin, liproprotein lipase, and glycerol-3-phosphate dehydrogenase and lastly for beige adipose tissue: transmembrane protein 26, tbox1 and short stature homeobox 2.37 The transformation of the beige adipose tissue from large lipid droplets and lack of UCP-1 content into multiple small, multi-locular droplets with high UCP-1 concentration is known as the “browning” of WAT. Nevertheless, this process is reversible. After the removal of the external stimulus, the beige adipose tissue will start a re-conversion into white like cell, a process known as “whitening”.38
Mitochondria are the main regulators of cellular energy. Reduced factors like FADH2 and NADH, obtained from macronutrients metabolism, provide the electrons needed to reduce O2 and form water molecules at the fourth complex of the ETC. Along with electron transportation through the ETC, protons are translocated from the matrix to the inner membrane space, thus, creating an increased electrochemical potential that will be later used to produce ATP by the ATPase. Although highly crucial, ATP production is not perfectly coupled leading to energy lost as heat by the re-entry of protons into the matrix, which will decrease the electrochemical potential. This proton leak is the result of two processes: a basal and an inducible leak. The inducible leak of protons is conducted by membrane proteins like UCPs (Figure 1).9
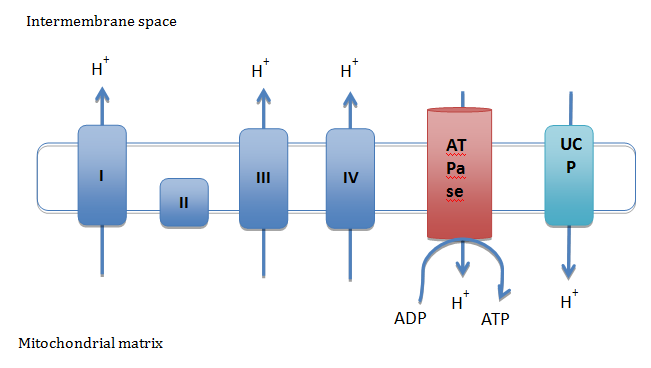
Figure 1 Induced proton leak through uncoupled proteins (UCP). UCP are proteins located in the mitochondrial inner membrane. While the electron transport chain carries protons from the matrix to the intermembrane space of the mitochondria, UCP transport protons in the opposite direction. The main function of UCP is to dissipate the electrochemical gradient to produce heat. Modified from Busiello et al.9
Uncoupling proteins are mitochondrial inner membrane anion carriers that decrease the electrochemical gradient in the inter-membrane space without an increase in ATP production. Futile protein conductance produces heat and accounts for 20 to 70% of the resting metabolic rate depending on the cell type.9,16,39 The first uncoupling protein discovered was UCP-1, which is predominantly expressed in BAT, accounting for as much as 10% of the mitochondrial proteins within this tissue. UCP-1 is overexpressed after cold exposure and overeating, having a half-life in the order ofhours to days.9 Its ablation in thermoneutral conditions is able to cause obesity even in mice fed a control diet.40 The second isoform, UCP-2, has shown to cause cytoprotection, immune cell modulation and regulation of glucose sensing in the brain and the pancreas, having a half-life of just 1h. Lastly, UCP-3 is mainly expressed in skeletal tissue but is also present in BAT and the heart, having a half-life of 1 to 4h. The described roles for this isoform include protection against ROS and increased FFA oxidation. Initially, it was thought that UCP-3 could play a role in thermogenesis since it is also induced by cold exposure; however, it was later discovered that its absence does not lead to a change in body temperature. Nonetheless, there is evidence that specific UCP-3 deletion from BAT leads to the impairment of the non-shivering thermogenesis. UCP-3 mutations have shown to be associated with significant changes in fat mass within humans.9,12 Transgenic over-activation of UCP-3 has shown to have exercise-like effects, increasing energy expenditure, causing complete fatty acid oxidation, and decreasing oxidative stress.41 Moreover, a positive association between metabolic rate and lifespan has been demonstrated in rodents. Mice in the upper quartile of energy expenditure showed 17% higher O2 consumption and 36% longer lifespan compared with mice from the lowest quartile. Mitochondria isolated from the group with the highest energy expenditure presented increased proton leak through UCP-3 and adenine nucleotide translocase.7 Another effect that UCP-3 has shown to regulate is glucose sensitivity. The protein levels of UCP-3 in the human vastus lateralis of patients with type II diabetes has been shown to be 50% of that from their healthy counterparts.16 UCP-3 activation is induced by different molecules including FFA, superoxide, and lipid peroxidation products. The expression of this mitochondrial protein seems to be controlled by PPARs, thyroid hormone, and the myogenic differentiation 1 protein, as well as by different conditions like fasting, cold exposure, and a high-fat diet.12 In conclusion, even though increasing thermogenesis might not be the main role of UCP-3 in the cell, its activation by the proper effectors can lead to thermogenesis.12
Peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC-1α) is a protein of 795 amino-acids with a molecular mass of 92 kDa that contains three phosphorylation sites for protein kinases. It is mainly expressed on brown adipose tissue, heart, kidneys and brain.13,38 PGC-1α is involved in a considerable number of cellular responses to external energy demanding factors. It controls body fat regulation, thermogenic response to cold conditions, mitochondrial biogenesis, and the expression of the electron transport chain, oxidative phosphorylation, and lipogenic proteins. Its deletion in mice is able to produce a significant increase in body fat percentage that is independent of food consumption or physical activity; moreover, null mice show lower muscle performance and exercise capacity.42 PGC-1α is able to co-activate the UCP-1 gene promoter by interacting with the transcription factors peroxisome proliferator-activated receptor alpha and gamma (PPARα and PPARγ).13,38,43
Peroxisome proliferator-activated receptors (PPARs) are transcription factors that locally regulate gene expression to satisfy metabolic demands and contribute with inter-organ communication.44 PPARα is a ligand activated transcriptional factor that belongs to the nuclear hormone receptor family. PPARα possess a wide range of metabolic effects depending on the expressing tissue. In skeletal muscle, a tissue with high PPARα expression, this transcription factor is able to switch substrate utilization from glucose to fatty acids, increasing their uptake and oxidation. In liver, besides increasing fatty acid uptake and oxidation, PPARα raises the production of high-density lipoprotein cholesterol while subsequently decreasing acute phase reactants and very low-density lipoprotein cholesterol. In starvation, PPARα is able to increase lipolysis in adipose tissue and fatty acid oxidation and insulin sensitivity in pancreas.44 Ureido-fibrate-5, a PPARα agonist that is 200-fold more potent than fenofibric acid, was able to decrease serum triglycerides by 70%, as well as inducing a significant increase in mitochondrial CPT-1 expression and β-oxidation in both liver and skeletal muscle.45 Furthermore, it has been demonstrated that PPARα null mice develop myocardial and hepatic steatosis, hypoglycemia, and an inadequate ketogenic response, which demonstrates the important metabolic effect of PPARα in regulating fatty acid oxidation, glycemic control, and the ketogenic response in the fasting condition.15 Besides its effect in fatty acid oxidation, PPARα has also shown to be able to control UCP-3 expression. WY 14643, a specific PPARα agonist, has shown to induce UCP-3 expression in a time- and dose-dependent manner, while thiazolidinediones, specific ligands for PPARϒ, does not show any effect in the expression of this mitochondrial protein. It is interesting to mention that WY 14643 treatment does not have any effect on FFA, indicating that, although FFA could play a role in UCP-3 expression, they are not the only mediators regulating its expression.46 Furthermore, it has been shown that AMPK could play a role in PPARα activation. As an example, the 5-aminoimidazole-4-carboxamide ribonucleotide, also known as AICAR, an activator of the AMP-activated protein kinase, has been shown to be able to increase the mRNA expression of PPARα and PGC-1α.47
A single bout of aerobic exercise increases the adipose tissue expression of PGC-1α, UCP-1 (adipose tissue), and UCP-3 (skeletal muscle), whereas training (3 weeks) does not seem to have an effect on their expression when enough time (12hours) is left between the last exercise bout and the sample collection.48,49 Interestingly, it has been proposed that exercise does not directly affect UCP-3 expression in skeletal muscle but rather due to the exercise-induced FFA release. In other words, if enough glucose supply is provided during physical activity, exercise does not have any effect on UCP-3 expression due to a blunted response in FFA levels.16 These findings could mean that the longer the exercise duration and the higher the intensity, the more pronounced the effect on skeletal UCP-3 expression could be, as long as energy supply is not being provided. However, further research in this area is necessary in order to elucidate the entire physiologic and metabolic pathway needed to activate UCP expression in both skeletal and adipose tissue.
β-aminoisobutyric acid (BAIBA), a small molecule produced by PGC-1α, is a myokine that has recently been shown to produce browning of white adipose tissue and increased β-oxidation in hepatocytes by acting through PPARα. In vitro, BAIBA treatment has shown to increase mitochondrial respiration and the expression of UCP-1 and cell death-inducing DFFA-like effector A (CIDEA) using cultured adipocytes from WAT. The results have been consistent in vivo, with BAIBA treatment increasing the expression of UCP-1, CIDEA, PGC-1α and cytochrome c in the same tissue. The above results were accompanied by improved glucose tolerance, increased whole body energy expenditure, and decreased body fat percentage, effects that occurred without significant modifications in activity or food intake. The complete metabolic pathway through which BAIBA acts has not been completely elucidated; however, it seems to act through PPARα. The before was corroborated when BAIBA treatment failed to increase the expression of thermogenic genes in WAT on PPARα null mice. Furthermore, it has been shown that three weeks of exercise training in rodents increases by 25 fold the gene expression of UCP-1 in WAT and by 5 fold the BAIBA concentrations in the gastrocnemius and quadriceps. These results were replicated in humans, finding an increase in BAIBA concentrations after 20weeks of exercise training (Figure 2). Lastly, BAIBA concentration in humans is inversely associated with metabolic risk factors.50

Figure 2 BAIBA effects on liver and adipose tissue. Exercise increases BAIBA release through PGC-1α activation in skeletal muscle. After its release, it travels through the circulation to target organs. BAIBA produces browning of the white adipose tissue and increases β-oxidation in the liver by acting through PPARα. Modified from Kammoun & Febbraio.51
Another mechanism of action that has been proposed for BAIBA’s effect is by acting through leptin. This hypothesis was proposed by conducting studies on ob/ob (a homozygous recessive mice with a mutation in the Ob gene that causes total leptin deficiency), on ob/+ mice (heterozygous mice that produces decreased leptin production), and wild type. BAIBA treatment in ob/ob mice failed to decrease body adiposity; however, it significantly decreased plasma HDL, hepatic necroinflammation, and the number of apoptotic nuclei in these genetically-modified mice. BAIBA treatment in ob/+ mice receiving a high-calorie diet has been shown to limit increases in body fat percentage by 40%. In addition, protective effects against such conditions as steatosis, necroinflammation, glucose intolerance, and hypertriglyceridemia were associated with elevated leptin levels. Additionally, ob/+ mice showed an increase in CPT-1 concentration in liver and WAT as well as an increased phosphorylation of acetyl-CoA carboxylase. Interestingly enough, the changes observed in ob/+ mice were present despite any significant changes in food consumption. Wild-type mice presented most of the effects observed in the heterozygous model, but in a less marked fashion (reduction of body fat mass of 27%). These results suggest that leptin may be a possible mechanism of action through which BAIBA performs its effects; however, the implication of leptin’s effects caused by BAIBA does not rule out the existence of other potential mechanisms of action since wild-type mice, with normal leptin levels, were also able to decrease body fat mass.51
The biochemical and molecular effects of exercise are now being better understood. Exercise, especially at higher intensities, increases energy expenditure even after the exercise session has finished. While aerobic exercise induces a robust, but transient increase in energy expenditure in the post-exercise period, resistance exercise seems to produce a slower, but longer increase in metabolic rate. One of the mechanisms through which exercise augments energy expenditure is by increasing the amount of BAT. UCP are mitochondrial proteins involved in thermoregulation. While UCP-1 is mainly expressed in BAT, UCP-3 expression is mainly expressed in skeletal muscle. UCP are regulated by the transcriptions factors PGC1α and PPARγ. Exercise has shown to be able to increase UCP-3 expression in skeletal muscle. Nonetheless, recent evidence has challenged the idea that exercise is the direct activator of this mitochondrial protein; postulating the increase in FFA caused by exercise as to the direct trigger. In this regard, it has been shown that nutrition in the pre- and post-exercise period plays a crucial role in controlling the gene expression activated by exercise, particularly affecting UCP expression in different organs. Novel research has discovered a pathway through which exercise influences PGC1α in skeletal muscle, which in turn increases the delivery of BAIBA to the circulation producing its effects in target organs such as BAT, where it acts through PPARα to activate UCP-1 expression. The identification of novel molecules with thermoregulatory properties such as BAIBA could potentially help to prevent or even to treat obesity. Further studies assessing the long-term regulation of factors controlling UCP expression are needed in order to evaluate its relevance on body composition.
None.
Author declares that there is no conflict of interest.

©2015 Marroquín, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.