Journal of
eISSN: 2373-4310


Review Article Volume 6 Issue 3
Department of Health, Human Performance and Recreation, Baylor University, USA
Correspondence: Samuel Frimpong, Professor and Robert H. Quenon Endowed Chair, Director, Heavy Mining Machinery Research Laboratory Member, APLU Board on Natural Resources, Missouri University of Science and Technology (Missouri S&T), Rolla, Missouri, USA, Tel +1-254-710-3243
Received: March 23, 2016 | Published: March 31, 2017
Citation: Marroquín FEM, Willoughby DS. Exercise and dietary factors affecting the micro biota: current knowledge and future perspectives. J Nutr Health Food Eng. 2017;6(3):67-73. DOI: 10.15406/jnhfe.2017.06.00199
Gut micro biota degrades complex polysaccharides and synthesizes short chain fatty acids that regulate the intestinal barrier by stimulating mucin synthesis. Mucin maintains the integrity of the gut by forming a mucus layer that covers and protects the intestinal epithelium. Lactate- and butyrate-producing bacteria maintain gut integrity in healthy individuals, while non-butyrate-producing, lactate-utilizing bacteria prevent optimal mucin synthesis. The effect of the micro biota on the host physiology was first observed with animal experiments involving fecal transplantation, in which impressive metabolic effects were passed from the donor to the host. These experiments showed that the micro biota from obese donors has an increased capacity to harvest energy from the diet, thus affecting body composition in the long term. One of the potential mechanisms through which the micro biota could affect body composition is by regulating the delivery of orexigenic and anorexigenic hormones, as it has been shown that the concentration of these hormones is correlated with several groups of the gut micro biota. Gut micro biota has not only been linked to body composition outcomes but also to the development and severity of certain metabolic abnormalities such as autoimmune diseases (type I diabetes and lupus), necrotizing enterocolitis, irritable bowel syndrome, ulcerative colitis, acute diarrheal infections, and frailty. It is well established that lifestyle factors such as exercise, diet, and supplementation with pre- and/or pro biotics affects the gut micro biota. The purpose of this review is to evaluate the effect of exercise, diet, and supplementation with pre- and/or pro biotics on the gut micro biota of humans and animals models. Clinical perspectives and future research areas will also be discussed.
Keywords: micro biota, micro flora, pre biotic, diet, exercise, gut bacteria
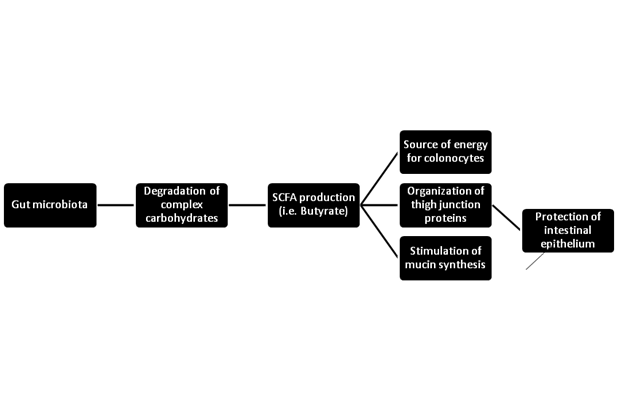
The gut microbial community is mainly formed by prokaryotic bacteria (99.1%), with only 0.1% from eukaryotic and viral origins. Bacteroidetes and Firmicutes constitute over 90% of the known Phylogenetic categories of the gut bacteria. The main functions of the micro biota are degradation of complex polysaccharides and synthesis of short chain fatty acids (SCFA).1 SCFA, such as butyrate, are produced by fermentation of undigested carbohydrates. These acids function as a source of energy for colonocytes that control the organization of tight junction proteins in the intestinal barrier and stimulate mucin synthesis.1‒3 Tight junction proteins, such as occluding, claudin-1, claudin-4, and ZO-1, form a par cellular seal in the intestinal lumen creating an epithelial barrier that protects the gut.4 Mucin is a glycoprotein that maintains the integrity of the gut by forming a mucus layer that covers and protects the intestinal epithelium against toxic agents (Figure 1).5 Lactate- and butyrate-producing bacteria maintain gut integrity in health individuals while non-butyrate-producing, lactate-utilizing bacteria prevent optimal mucin synthesis.6
The effects of the micro biota on the host physiology were first observed with experiments involving fecal transplantation, in which impressive metabolic effects were passed from the donor to the host.5 These first experiments demonstrated that micro biota from obese donors has an increased capacity to harvest energy from the diet (increased Firmicutes to Bacteriodetes ratio), which is able to affect body composition in the long-term. This conclusion was made after performing fecal transplantation from ob/ob mice (obese mice due to mutation in leptin gene) into lean, germ-free mice, resulting in a significant increase in fat percentage without concomitant increments in energy consumption.5 It is important to highlight that lean, germ-free mice acquired the micro biota of the donor ob/ob mice. The fact that Bacteriodetes concentration has shown to increase in weight loss highlights the potential importance of the Firmicutes to Bacteriodetes ratio in regulating body composition (Figure 2).7 Furthermore, orexigenic and anorexigenic hormones have shown to be significantly correlated with several groups of the gut micro biota. Leptin positively correlates with Bifidobacterium and Lactobacillus, with a significant negative correlation with Clostridium, Bacteroides and Prevotella. Ghrelin, on the other hand, is positively correlated with Bacteroides and Prevotella, while negatively correlated with Bifidobacterium, Lactobacillus and B. Coccoides-Eubacterium rectale group.8 This correlation might be a potential mechanism of action through which the micro biota affects body composition; nonetheless, research in this area is still far from conclusive.

Micro biota has not only been linked to body composition outcomes but also to the development of certain metabolic abnormalities such as autoimmune diseases and frailty. Prevotella and Akkermansia, butyrate-producing and mucin-degrading genera, are 20- and 140-fold higher in heathy controls compared to patients with type 1 diabetes, whereas patients with type I diabetes have higher Bacteroides, Veillonella and Alistipes concentration in comparison with healthy controls. These later bacteria ferment glucose and lactate to propionate, acetate and succinate, which (unlike butyrate) do not induce mucin synthesis.4 Fecal micro biota composition has also shown to have a strong relationship with frailty. Frailty is the diminished reserve in one or more of the following components: mobility, physical fitness, comorbidity, weight loss, vision, hearing, cognition, and psychosocial resources. Fecal samples from subjects with high frailty scores show a significantly low concentration of lactobacilli (26-fold), Bacteroides/Prevotella (3-fold) and Faecalibacterium prausnitzii (4-fold) with high concentrations of Enterobacteriaceae (7-fold). Additionally, a significant negative correlation (correlation coefficient: -0.694, p<0.0001) was found between lactobacilli concentration and frailty score9 indicating a potential beneficial effect of lactobacilli in the different components of frailty.
The effect that environmental factors have on the gut micro biota has received special attention in the last couple of years.9‒10 This review will evaluate the effect of exercise, diet, and supplementation with pre- and pro biotics, both individually, as well as in combination, on the gut micro biota of humans and animals.
Using the online database PubMed, full text articles were obtained by using the terms “exercise and micro biota”, “diet and micro biota”, “prebiotics and micro biota”, and “prebiotics and micro biota”. Articles were initially screened according to their title and abstract; careful evaluation of the full manuscripts was then used to identify relevant articles. Recent articles including animal and human studies, as well as normal to overweight/obese populations were selected. Complementary references were obtained from the previously-selected articles.
It is well established (at least in animal models) that exercise affects the micro biota. Exercise training in horses has shown to affect the gut micro biota at the phyla level. Aerobic training (3min/day, 6d/week, for 42days) causes significant changes in the micro biota that are more noticeable in the first stages.11 Exercise has also shown to alter the composition of the micro biota at the genus level in rats. A recent animal study analyzed the effect of training 30min per day, 5days per week for 4weeks on the gut micro biota composition. While control (Wistar) and hypertensive rats (spontaneous hypertensive rats from breeding of Wistar-Kyoto rats with high blood pressure) shared a similar micro biota profile, obese rats (fa/fa Zucker rats) had a divergent micro biota composition. Exercise training increased Allobaculum (hypertensive rats), Pseudomonas (Obese rats) and Lactobacillus (obese rats), while decreased Streptococcus (Wistar rats), Aggregatibacter (Hypertensive rats) and Sutturela (hypertensive rats).12
Interestingly, research suggests that exercise and the micro biota have a bidirectional affect, which means that exercise is able to affect the micro biota, and the micro biota itself affects exercise performance. This implication would place exercise and the micro biota in a vicious circle where each of them mutually affects one another. The rationale behind the idea of having the micro biota regulating exercise performance states that the micro biota helps promote digestion and food absorption, which can potentially affect energy utilization during exercise. Germ-free mice have a 2-fold decrease in exercise performance in comparison with specific pathogen-free mice. Germ-free mice have lower liver, muscle, brown adipose tissue, and epidemical fat weights in comparison with specific pathogen-free mice. This could indirectly indicate a lower capacity of the germ-free mice to harvest and store energy from glycogen and triglyceride. Besides showing a decrease in exercise performance, the germ-free mice have lower levels and activity of the antioxidants glutathione peroxidase and catalase in both serum and liver, which can become detrimental in exercise performance. Monocolonization of germ-free mice with Bacteroidesfragilis (bacteria with beneficial effects on host homeostasis) prevents the decline in exercise performance and increased glutathione peroxidase and catalase activity,13 two important enzymes involved in antioxidant reactions.
Although promising animal data is available, human studies analyzing the effect of exercise on the gut micro biota is still scarce. A cross-sectional study compared the effect of exercise training on the gut microflora by comparing trained rugby players (experimental group) with two control groups (one matched for athlete size (high BMI), while the other matched for age and gender (low BMI)). The study showed that the experimental group had significantly higher concentrations of gut micro biota diversity and creatine kinase (indicator of muscle damage), while having lower inflammatory status. The top six taxa changes in athletes relative to the size-matched group were Firmicutes, Ruminococcaceae, S24-7, Succinivibrionaceae, RC9 and Succinivibrio groups. The top six taxa changes in athletes relative to age and gender matched controls were Prevotellaceae, Erysipelotrichaceae, S24-7, Succinivibrionaceae, Prevotella and Succinivibrio groups. Additionally, the experimental and the low BMI group had significantly higher proportions of the genus Akkermansia in comparison with the high BMI control group.14 Akkermansiamuciniphilla is a mucin degrading bacteria that resides in the mucus layer. Its abundance is inversely correlated with obesity in mice and humans.15,16
Although moderate exercise seems to have a positive effect in the micro biota, intense, prolonged endurance exercise has shown to generate a condition known as “leaky gut”. Leaky gut involves loosening of the tight junction proteins, increasing intestinal permeability and risk for infections.17
Diet has also been shown to influence micro biota composition which determines the amount and composition of SCFA present in the gut. Previous research has shown that the lack of enteral nutrition increases the concentration of bacteria that uses mucus as a substrate. This is because mucus is now the only source of food available, thus total parenteral nutrition debilitates the intestinal mucosal layer and decreases the intestinal protection18 C. perfringens, has shown to be opportunistically enriched in total parenteral nutrition.18
A 6week intervention with a very low energy diet (800kcal), high in protein and low in carbohydrates and fat, modified the micro flora of obese individuals. At the end of the 6weeks, all the studied bacterial groups tended to decrease with exception of Bacteroides spp, which increased. This result might be caused due to the considerable high protein content of the diet, as Bacteriodes are a predominantly proteolytic species. From the bacteria that decreased, Bifidobacteria showed the most drastic decrease followed by Lactobacillus. The low intake of carbohydrates likely reduced the substrate for Bifidobacteria and Lactobacillus group, thus decreasing their concentration.19
Exercise in combination with food modifications can alter the composition of the gut micro flora. A four arm study analyzed the differential effect of both exercise and food restriction on the gut micro flora for a period of 6 days. The groups included: food restriction with free access to exercise, food restriction without access to exercise, food adlibitum with free access to exercise, and food restriction without access to exercise. Caloric restriction caused an overall significant increase in the number of Proteobacteria, Bacteroides, Clostridium, Enterococcus,Prevotella and Msmithii, with a significant decrease in the quantities of Actinobacteria, Firmicutes, Bacteroidetes, B.coccoides-E rectale group, Lactobacillus and Bifidobacterium with respect to unrestricted eaters. Exercise intervention on the other hand caused overall a significant increase in the number of Lactobacillus, Bifidobacterium and B. coccoides-E. Rectal.7 The increment in Bifidobacteria and Lactobacillus observed in the exercise group might be beneficial as it has been previously demonstrated that these two bacteria groups have the capability of forming lactate, which is later converted into butyrate by butyrate-producing bacteria in the gut.4
High fat diet (HFD) induces metabolic and gut microbial changes.20‒22 Although, exercise has shown to effectively reverse some of the detrimental metabolic effects of a HFD, the potential effect of exercise restoring the altered micro biota caused by HFD is still on debate. An 8week, four-arm study analyzed the effect of HFD (60% of calories from fat) and exercise (1h/session, 6d/week for 8weeks at a speed of 7m/min) on gut diversity. By using four groups (HFD with exercise, HFD without exercise, normal diet with exercise, and normal diet without exercise), the effect of exercise and diet on the gut micro biota was isolated. The results demonstrate that HFD drastically changed the gut microbial community. Similarly, exercise was also able to massively change the micro biome; however, the changes induced by exercise were independent from the effect caused by diet. In other words, exercise was not able to rescue the changes caused by a HFD in the gut micro biota.23 The clinical significance of this finding is still poorly understood.
In contrast to the previous study, Denou et al.,21 found that exercise training was able to successfully overcome the changes caused by HFD in the gut micro biota. Two initial groups (control and HFD) were kept until 8weeks of age, when the HFD (45% calories from fat) group was subdivided into exercise and non-exercise interventions. Exercise consisted of high intensity interval training in the treadmill (17m/min for 2 min at a 5% rate, with resting intervals of 2min each) for a period of 1h/day, 3d/week for 6weeks. The HFD decreased the Bacteroidetes-to-Firmicutes ratio. However, at the end of the 6weeks of exercise, an increase in the Bacteroidetes-to-Firmicutes ratio was observed, despite no significant changes in body composition. Aerobic exercise was also able to increase the alpha diversity of the gut micro biota. According to these results, exercise is able to revert the changes caused by a HFD; however, body composition seems not to be a driving factor controlling the effect of exercise on the gut micro biota.24
A more recent study supported the results found by Denou et al.,21 showed exercise to efficaciously combat HFD-induced microbial changes while improving intestinal health. The study analyzed the effect of HFD (45% calories from fat) and exercise for 12weeks through the use of four groups: HFD with exercise, HFD without exercise, normal diet with exercise, and normal diet without exercise. The study demonstrated that independent of the diet, both exercise groups (lean and HFD) had lower cyclooxygenase-2 expression (inflammation-related enzyme) and ghrelin (orexigenic) and IL-6 concentrations, while having higher peptide YY (anorexigenic) and pancreatic polypeptide (anorexigenic) levels compared with the non-exercise groups. Despite the higher caloric consumption of the HFD-exercise group, they had lower fat mass and total body mass compared to their HFD-sedentary counterparts. Faecalibacterium prausnitzi was only detected in exercised animals, while animals in the normal diet had higher Lachnospiraceaespp that were not present in the HFD animals. Exercise in combination with a normal diet increased Allobaculum spp and Clostridium spp, while exercise in combination with a HFD resulted in increased Peptococcus spp.25
The difference between these three studies could have been caused mainly due to variations in some of the variables used in their methodologies. The type of diet differed among the studies, the study performed by Kang et al.,20 utilized a 60% fat-based diet, while the diets provided by both Denou et al.,21 Campbell et al.,22 consisted on a 45% fat-based diet. The studies that involved the diet with 45% of energy coming from fat were able to revert with exercise the effects in the micro biota caused by HFD, while the study utilizing a higher percentage of fat (60%) was not able to observe this restoration. The type of training protocol is the second variable that could have contributed to the differential results. The study performed by Kang et al.,20 used continuous aerobic exercise (7m/min, 1h/day, 6d/week for 8weeks), the study by Denou et al.,21 involved high intensity interval training (17m/min for 2min at a 5% rate with 2min resting intervals, 1h/day, 3d/week for 6d/week), while the study by Campbell et al.,22 involved free wheel running (12weeks). The intensity of the workout might have a different effect in the oxygen provision to the colon which has shown to affect the kind of bacteria that is able to survive (aerobic versus anaerobic bacteria).
Research continues to take place in this area and soon studies will be able to reveal more of the effect of lifestyle interventions on the micro biota. A 6month randomized intervention analyzing the effect of aerobic exercise in combination with a low carbohydrate diet on non-alcoholic liver disease (NAFLD) subjects has been taking place from 2013 to 2015. Recruited participants are expected to perform supervised individualized exercise training (30-60min per session) 3-6times per week, while maintaining a low carbohydrate diet (<30-40%). The publication of this study will provide us with a deep insight into the effect of lifestyle interventions on NAFLD subjects potentially via modification of the gut microflora.26
Pro biotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”.25 Prebiotics are defined as non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of a limited number of bacterial species already established in the colon.27 Although acting through a different mechanism, both pre- and pro biotics improve health by modulating the bacterial composition in the gut.24
Lactobacillus gasseri is located within the genus of Lactobacillus acidophilus and is a well-known probiotic and critical component of the gut flora. Twelve weeks of supplementation with Lactobacillus gasseri BNR17 (isolated from human breast milk) has shown to decrease weight gain in rats following a HFD without altering food consumption. Additionally, the authors observed that rats within the probiotic group were protected from morphological alterations and increments in liver weight caused by the HFD.28
Supplement consumption has also shown to beneficially affect autoimmune diseases through changes in the micro biota. Lupus-prone mice (MRL/Mp-Fas1pr) have different bacterial composition and diversity compared to healthy controls. Lupus prone mice presented Lactobacilli depletion with increased Lachnospiraceae, Ruminococcaceae and Rikenelaceae concentration in comparison to controls. Supplementation with retinoic acid (6mg/kg of body weight) restored Lactobacilli concentration to normal values, which correlated with improved symptoms. It is important to highlight that supplementation with retinol, the primary ingredient in most vitamin A supplements, increased even more the fraction of Lachnospiraceae in the gut of Lupus-prone mice.29 Careful supplementation with retionoic acid-specific supplements is recommended.
Prebiotic treatment has also shown to be beneficial. Oligo fructose supplementation for 4weeks has shown to normalize Akkermansia muciniphila concentration in diet-induced obesity. The restoration of Akkermansiamuciniphila was significantly and inversely correlated with fat mass. Moreover, specific Akkermansiamuciniphila supplementation reversed the fat-mass gain, metabolic disorders, metabolic endotoxemia, adipose tissue inflammation, and insulin resistance observed in HFD mice. The decrease in mucus thickness observed in obesity increases the gut permeability. Despite the fact that Akkermansiamuciniphila forms part of the mucin-degrading family, its supplementation restored the 46% decrease in mucus thickness observed in HFD.15
A symbiotic supplement that combines both pro biotics and pre biotics is thought to yield greater efficacy than the use of either of them alone. A double-blinded, parallel-controlled trial analyzed the effect of a symbiotic (n=11) versus a pre biotic supplement (n=11) on active individuals for a period of 21days. The symbiotic supplement contained four pro biotics, two pre biotics, as well as bovine whey-derived lactoferrin, and immunoglobulins, while the pre biotic only contained Acacia gum. At the end of the study, a 9-fold increase in Lactobacillusparacasei in the symbiotic group in comparison with the prebiotic group was observed. No significant effect on SCFA, Lactobacilli, L acidophilus, L. rhamnosus, B. lactis and E. coliwere observed. A lower increase in the pro-inflammatory cytokine IL-16 (chemotactic for immune cells, particularly T-cells) was observed in the symbiotic group in comparison with the pre biotic group. Also, no significant effect on gut permeability was noted. The evidence shows that the consumption of 3 pills per day was probably not enough to produce significant alterations in the gut micro biota, an effect that was evidenced by the lack of a significant increment in SCFA. Whether the increase in Lactobacillusparacasei is of clinical importance remains to be further evaluated. It is also a possibility that significant alterations in both the micro biota and the gut permeability were not noted as the participants were healthy populations perhaps the symbiotic could have a more pronounced effect on subjects with altered micro biota or permeability.30
Heavy exercise training often produces episodes of illness, which could interrupt the training sessions and prevent athletes from achieving maximum performance during competitions.31 Supplementation with Lactobacillusfermentum (109 CFU) for 11weeks decreased symptoms of lower respiratory illness (coughing with chest congestion and/or wheezing), gastrointestinal (GI) problems (nausea, vomiting, flatulence, stomach rumbles and loss of appetite), and use of cold or flu medication in well-trained male cyclists. In contrast, there was a significant two-fold increase in the number and duration of self-reported symptoms of lower respiratory illness in females taking the probiotic. The difference in gender might mean that females need higher doses of pro biotics than males to exert the same effects. Another potential explanation is that different bacteria strains have different effects on the micro flora of males versus females; however, these hypotheses have not been tested. For both males and females taking the probiotic supplementation there was a significant increase in self-reported GI symptoms, which can reflect adaptation of the gut to the alteration of the micro flora composition.30 It has been previously demonstrated that the initial stages of probiotic supplementation can develop abdominal bloating, flatulence, and cramping the first two weeks of supplementation,31 however, the GI symptoms developed by the participants involved in this study maintained their GI symptoms during the 11weeks of the supplementation. It is important to keep in mind that prospective studies evaluating the effect of exercise training and/or probiotic supplementation on GI symptoms and lower or upper respiratory infections are typically associated with having a low rate of illness appearance, which decreases the power of the study.32
The majority of the studies have analyzed the effect of chronic supplementation of pro biotics; however, it seems that pro biotics might also posses acute effects. The supplementation of Lactobacillus paracasei (1010 CFU) in a normal to overweight population showed to significantly decrease food intake in the meal following the consumption of the probiotic 4h afterwards. The exact mechanisms could not been isolated as hunger, fullness, and satiety, and postprandial insulin and GLP-1 concentrations were not affected. More intriguingly, after separating by gender, a significant decrease in food intake was present but only in males.32 As mentioned previously, these results might also imply the need for a higher probiotic dose in female subjects in order to exert the same effects observed in males. Another potential explanation could be a differential effect of microbes on female metabolism.
Micro biota research is still in the early stage in science, and involves mainly descriptive studies. The implication and interpretation of changes in the micro biota caused by lifestyle interventions are not yet fully understood. Some of the studies evaluating the gut micro biota are correlational, which implies that cause and effect cannot be assigned. Furthermore, although certain strains of micro biota have shown to be beneficial for improvements in body composition, a recent publication demonstrated that micro biota depletion is able to decrease subcutaneous and visceral adipose tissue in mice. This decrease in adipose tissue was accompanied by increments in food intake and conversion from white into beige adipocytes [corroborated by increments in uncoupling protein-1 (UCP-1) content]. Browning of the white adipose tissue was associated with improved glucose tolerance, and insulin sensitivity in lean, ob/ob and HFD mice. The browning process was present as soon as 10days after micro biota antibiotic-induced depletion and further increased after 40-60days.33 Whether this effect is conserved in the human species remains to be discovered.
Nonetheless, beneficial effects of the gut micro biota have been demonstrated on necrotizing enterocolitis, acute diarrheal infections, ulcerative colitis, and irritable bowel syndrome.34 A shift from obligate anaerobes (Faecalibacteriumprausnitzii) to facultative anaerobes (Enterobacteriaceae) takes place in the elderly and in inflammatory bowel diseases, thus, favoring pathogenic bacteria and inflammation.8,35 Gut micro biota affects health status by modifying vitamins absorption (vitamin B7 and B12), creatine, and carbohydrate metabolism.36,37 A change in the diet from low-fat, low-sugar, towards a high-fat, high-sucrose diet has shown to be related with an unhealthy gut micro biota composition.38 The oxygen content in the ileum seems to also play an important role in micro biota composition. The distal portion of the gastrointestinal tract from healthy humans is characterized by low levels of oxygen. An increase in oxygen concentration in the ileum has shown to promote a shift from obligate anaerobes to facultative anaerobes, thus promoting dysbiosis.35 Dysbiosis involves a reduction in the diversity of the gut micro biota as well as a decrease in obligate anaerobes, with an increase on facultative anaerobes and even aerobes. If the oxygen hypothesis proves to be right, oral supplementation of pharmacological agents, such and antioxidants or pro biotics with great ability to capture oxygen, could be an effective line of treatment for inflammatory bowel disease and other comorbidities.35
Interpretation of the metabolic effect of specific strains of gut bacteria is difficult, as available evidence is still far from conclusive. Until now, it seems that the Bacteroidetes-to-Firmicutes ratio has an important role in the effect of the gut micro biota on body composition. Ob/ob mice and mice following a HFD have a decreased Bacteroidetes-to-Firmicutesratio, while weight loss and aerobic exercise training has resulted in an increase.
Aerobic exercise increases gut diversity, which has been liked to improvements in health status. Exercise has also shown to increase the concentration of Lactobacillus, Bifidobacterium, B. coccoides-E. rectale, Faecalibacterium prausnitzi, Akkermansiaceae and Akkermansia. While caloric restriction, on the other hand, has shown to increase Bacteroidesspp, Proteobacteria, Clostridium, Enterococcus, Prevotella and M smithii.
Supplementation with different species of Lactobacillus has shown to decrease weight gain in rats following a HFD (Lactobacillusgasseri), decrease symptoms of lower respiratory illness (Lactobacillus fermentum), and decrease food intake in the meal following the consumption of the supplement (Lactobacillus paracasei). Prebiotic treatment has also shown to be beneficial. Oligo fructose supplementation normalizes Akkermansiamuciniphila concentration, which has been shown to inversely correlate with obesity and metabolic disorders in mice and humans.
Micro biota diversity might become a new marker of health status as it seems to play a key role in body composition and the development of gastrointestinal and autoimmune diseases. As mentioned by Turnbaugh and his research team: “If the gut microbiome of obese humans is comparable to that of obese mice, then it may be a biomarker, a mediator and a new therapeutic target for this increasingly worldwide disease”.5 Some cautions should be taken when recommending supplementation in the clinical setting, as the properties from a subcategory of a probiotic should not be extrapolated to another one. Additionally, the concentration of the probiotic provided in a study with promising results should be conserved in the clinical practice, respecting also the duration of the intervention that took place in the specific scientific study.
Longitudinal human research is needed in order to show the cause-effect of lifestyle factors and to provide information on the time needed to observe beneficial effects. Moreover, studies comparing the effect of different exercise modalities on the micro biota will provide information on whether there is an exercise modality that could be more beneficial to positively alter the micro biota. Lastly, the effect of combined exercise and either diet, pro biotics or pre biotics, on human’s micro biota has not been assessed. Therefore, whether there is a negative or synergistic effect still remains to be elucidated. Involving special populations and replicating in vivo the outcome of in vitro studies is greatly encouraged. Both authors accept responsibility for the content of this review. No conflict of interests is present.
None.
Author declares that there is no conflict of interest.

©2017 Marroquín, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.