Journal of
eISSN: 2373-4310


Research Article Volume 12 Issue 1
Department of home sciences and management, Faculty of agriculture, Federal University Gashua, Nigeria
Correspondence: Ogori AF, Department of home sciences and management, Faculty of Agriculture, Federal University Gashua, Nigeria
Received: December 10, 2021 | Published: February 4, 2022
Citation: Ogori AF. Effects of thermal processing on edible seed plant proteins. J Nutr Health Food Eng. 2022;12(1):14-21. DOI: 10.15406/jnhfe.2022.12.00349
This chemistry, structure and the effects of processing methods on functionalities and significance of plant proteins was reviewed. Plant seed proteins are known to provide various beneficial activities and are essential source of amino acids; peptides fractions as extended functional units. These extended moieties enhances nutritional and functional properties of food and food systems. The review elaborates on structure of cereal, legume and vegetable plant proteins and their functional outcomes, nutritional properties and challenges associated during processing approach.
Keywords: chemistry, structures, plant protein, functionality, processing methods
Proteins are highly complex substances that are present in all living organisms. Proteins are of great nutritional value and are directly involved in the chemical processes essential for life. Food proteins outside fighting diseases as antibodies and part of many intracellular systems as well for reproduction, makes food sensorially acceptable. Protein molecule is very large compared with molecules of sugar or salt and consists of many amino acids joined together to form long chains, much as beads are arranged on a string.
Protein concentrate and isolates enhance the nutritional values of food and food systems. Besides modification of foods,1 they add value to traditional foods like tubers and roots, meat and cereal products . Certain functionality and for production of novel items like meat analogue, fish and milk substitutes.1
There are about twenty different amino acids that occur naturally in proteins. Proteins of similar function have similar amino acid composition and sequence. Although it is not yet possible to explain all of the functions of a protein from its amino acid sequence, established correlations between structure and function can be attributed to the properties of the amino acids that compose proteins. Plants can synthesize all of the amino acids; animals cannot, even though all of them are essential for life. Plants can grow in a medium containing inorganic nutrients that provide nitrogen, potassium, and other substances essential for growth. They utilize the carbon dioxide in the air during the process of photosynthesis to form organic compounds such as carbohydrates making them unique sources of protein.
Plant protein source include legumes soybean, green plant folders like alfalfa , cereal wheat , nut and seeds , these could be processed to leaf protein isolate, concentrate, isolates and hydrolyzates.2 The seeds of legumes are increasingly being used to prepare inexpensive protein-rich foods.
Classifications of amino acids3
Experts classify amino acids based on a variety of features, including whether people can acquire them through diet. Accordingly, scientists recognize three amino acid types:
Classification based on nutrition and physiological functions
Histidine is an amino acid that is categorized as semi-essential since the human body does not always need it to properly function therefore dietary sources of it is not always essential. Meanwhile, conditionally, essential amino acids are not usually required in the human diet, but do become essential under certain circumstances.
Asparagine, Alanine, Arginine, Aspartic acid, Cysteine, Glutamic acid, Glutamine Proline, Glycine Tyrosin, Serine.
Classification based on functional group structures
This depends upon the side chain and shape structures, and experts recognize these five as:
Sulphur containing Amino Acid- Cysteine and Methionine
Neutral amino acids - Asparagine, Serine, Threonine and Glutamine
Acidic AA - Glutamic acid and Aspartic acid
Basic AA- Arginine and Lysine
Aliphatic amino acids - Leucine, Isoleucine, Glycine, Valine and Alanine
Aromatic amino acids - Phenylalanine, Tryptophan, and Tyrosine.
Classification based on polarity and side chains
Non-polar side charged chains - Phenylalanine, Glycine, Valine, Leucine, Alanine,
Isoleucine, Proline, Methionine and Tryptophan
Polar side charged chains - Tyrosine, Serine, Asparagine, Threonine, Glutamine, and Cysteine
Charge side chain - Aspartic acid and glutamic acid, lysine and Arginine.
Other classifications
Simple protein - Are amino acid that have simple peptides
Conjugated protein- Amino acids that have that have simple fraction with prosthetic group for example pigment lipoproteins.
Derived protein - Amino acid derived from acid and alkaline hydrolysis for example peptides, and polypeptides.
Properties and structures of plant protein
Zwitterions structure
The common property of all proteins is that they consist of long chains of α-amino (alpha amino) acids. The general structure of α-amino acids is shown below. The α-amino acids are so called because the α-carbon atom in the molecule carries an amino group (-NH2); the α-carbon atom also carries a carboxyl group (-COOH).
In acidic solutions, when the pH is less than4, the -COO groups combine with hydrogen ions (H+) and are thus converted into the uncharged form (―COOH). In alkaline solutions, at pH above 9, the ammonium groups (-NH+3) lose a hydrogen ion and are converted into amino groups (-NH2). In the pH range between 4 and 8, amino acids carry both a positive and a negative charge and therefore do not migrate in an electrical field. Such structures have been designated as dipolar ions, or zwitterions (i.e., hybrid ions).
Peptide structure
Peptides are formed by bonding of amino acid together via amino acid links during hydrolysis (acid/alkaline) activity on the chains of plant protein releasing peptides.
Although more than 100 amino acids occur in nature, particularly in plants, only twenty types are commonly found in most proteins. In protein molecules the α-amino acids are linked to each other by peptide bonds between the Amino group of one amino acid and the carboxyl group of its neighbor.
The condensation (joining) of three amino acids yields the tripeptide.
Peptides types
Glutathione
Is peptides found in many plant foods (glutein, cysteine, + glycine). It involves the rheology of wheat flour that brings about reduction of disulphid bond and reduce molecular weight of plant proteins.
Lysine peptides
This retard the bond reaction with glucose, here it is good for lysine fortification or sugar coating food which must be heated.
Physical Structure and Reaction of protein
Dissociation reaction
Protein when in solution has ability to dissociate its ions and these gives its ability or functionality for solubility. In solution protein form zwitterion ability.
Optical activity/ configuration
Except glycine all amino acids have its optical and chiral centers for mirror images at α – C – atom(Cahn In gold Prelog system of classification as N -and S- amino –acids.
Solubility
Solubility depends on the structure of amino acid. However certain plant protein are specific to solubility such as proline, hydroxlproline, glycine and alannin are all soluble in water. Cysteine tryrosine are less soluble. See above
Structural stereochemistry of amino acids
The amino acids present in proteins differ from each other in the structure of their side (R) chains. The simplest amino acid is glycine, in which R is a hydrogen atom. In a number of amino acids, R represents straight or branched carbon chains. One of these amino acids is alanine, in which R is the methyl group (-CH3). Valine, leucine, and isoleucine, with longer R groups, complete the alkyl side-chain series. The alkyl side chains (R groups) of these amino acids are nonpolar; this means that they have no affinity for water but some affinity for each other. Although plants can form all of the alkyl amino acids, animals can synthesize only alanine and glycine; thus valine, leucine, and isoleucine must be supplied in the diet.
Two amino acids, each containing three carbon atoms, are derived from alanine;
Alanine are serine and cysteine. Serine contains an alcohol group (-CH2OH) instead of the methyl group of alanine, and cysteine contains a mercapto group (-CH2SH). Animals can synthesize serine but not cysteine or cystine.
Cystine
Consists of two cysteine molecules linked by the disulfide bond (―S―S―) that results when a hydrogen atom is removed from the mercapto group of each of the cysteine. Disulfide bonds are important in protein structure because they allow the linkage of two different parts of a protein molecule to and fro thus the formation of loops in the otherwise straight chains. Some proteins contain small amounts of cysteine with free sulfhydryl (-SH) groups.
Four amino acids
Each consisting of four carbonatoms, occur in proteins; they are aspartic acid, asparagine, threonine, and methionine. Aspartic acid and asparagine, which occur in large amounts, can be synthesized by animals. Threonine and methionine cannot be synthesized and thus are essential amino acids; i.e., they must be supplied in the diet. Most proteins contain only small amounts of methionine.
Glutamic acid
Proteins also contain an amino acid with five carbon atoms (glutamic acid) and a secondary amine (in proline), which is a structure with the amino group (-NH2) bonded to the alkyl side chain, forming a ring. Glutamic acid and aspartic acid are dicarboxylic acids; that is, they have two carboxyl groups (-COOH).
A stabilizer of hydrogen ion concentration) by binding hydrogen ions (H+) to the nitrogen atoms of the imidazole ring.
Reaction of amino acid
Here methionine will reduce sugar in strecker degradation for flavor formation. Lysine and threonine can loss their biological value via isolation.
Peptides can also be synthesized via acylation process
Reaction involving other Functional amino acid groups
Lysine- selective acylation of e- amino group during acylation of CU2+ complex
Aspatic/glutamic acid – alkalized catalyzed hydrolysis of metal and ethyl esters.
Aspartic and glutamic acid bond to peptide can result in formation of iso-peptides
Serine and threonine- acidic hydrolysis can yield α- keto acid via elimination of water.
Amino acid structure
The functionality of protein formed from amino acid sequence is dependent on its structure which determines the molecular conformation. Amino acid, the functional entity of protein is sequenced via synthesis.
N - Terminal chain can be removed by hydrolysis
C - Terminal chain can be removed by enzymatic hydrolysis
The effect of heat, and other processing activities during applied food processing results in conformation, configuration via S-S, hydrogen cleavages and bending exposing reactive side chains.
Primary structure
The primary structure of a protein is determined by its amino acid sequence without any regard for the arrangement of the peptide chain in space. Its depends on the sequence of amino acid in the chain.
Secondary structure
The secondary structure gives the spatial arrangement of the main peptide chain in the space, No regard for the conformation of side chains or other segments of the main chain.
But having helix, spiral and random pleated shapes.
Tertiary structure
The tertiary structure is characterized by both the side chains and other adjacent main chain structures. The nature of the protein confers interaction using H-bonding, S-S- as a result of folding either into helix pleaded or random allowing large peptides folding. Some of them contain positively or negatively charged groups, others are polar, and still others are nonpolar. Positively and negatively charged side chains have the tendency to attract each other; hence hydrophobicity and hydrophilic characteristics.
Source: Benitze et al3, Ihekoronye and Ngoddy.4
Quaternary structure
Quaternary structure is used for the arrangement of identical or different subunits of large protein peptides in which each subunit is a separate peptide chain. In plant proteins, the subunits are bound to each other by covalent bonds (disulfide bridges), hydrogen bond. The stability here is brought by polarity and degree of exposure to aqueous environment.
Source: Ihekoronye and Ngoddy.4
Reaction of amino acids at high temperature
Denaturation
This could also occur during high temperature process. This happen during dry heat process of cereals legumes and certain fiber process. The conformational change exposes the bond chain leading to hydrogen bonding breaking of S-S links.
Cross linking effect
Cross linking between proteins is achieved with transglutinnas and peroxidase. This is when fermentation of tubers with amylase or lyased emzymes are involved. Incubation of protein with peroxidase,hydroden peroxide, catechol3 also result in cross linking. The result is deamination of lysine residue followed by aldo and aldamine condensation- (maillard reaction) to make new protein – plasteins. Plastains helps in some physiological activities in the body.
Effect of unite processing on plant proteins
The chemical nature and characteristic of protein from plant are significant. Heat processing method such as a radiation, immersion cooking, steaming process, baking, fermentation, wet cooking, soaking and roasting have great impact on the legume, cereal, nut and seeds produce and product due to that this method changes the conformations of the tertiary and quaternary structures of these proteins found in these food materials.
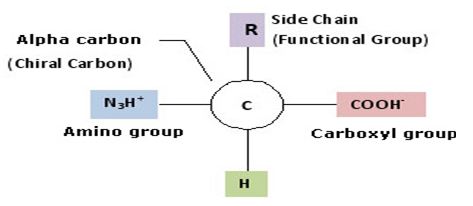
Figure 1 An amino acid structure.3
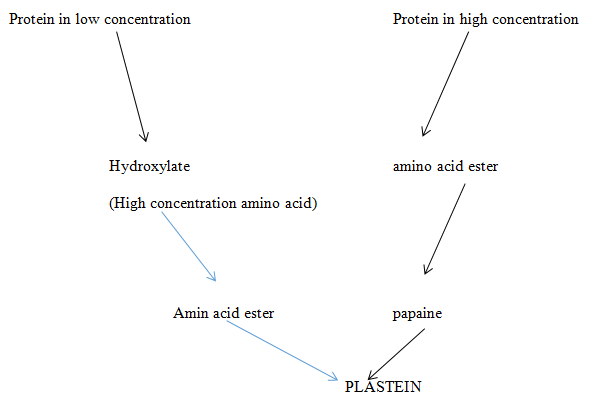
Figure 2 Cross linking producing plastein.3
Direct thermal processing such as roasting frying grilling however makes food palatable and easy to digest but nutritionally does not support protein bioavailability , its make vitamins and precursors unavailable and triggers binding of other food nutrient during Millard reaction and others. The nutrient value of protein is increased during cooking. Onwuka5 had reported low crude protein content and not quality profile of amino acid in maize and cowpea, with favorable digestibility.
Extrusion
Extrusion method which entails cooking, extrusion, pasteurization and drying may impair texture and nutrient quality of protein.6 Extrusion changes order protein conformation to randomized unfolded structure by disrupting non covalent bounds exposing more polar groups and or non polar group which has desirable and undesirable outcome depending on process in which it is subjected to.
Baking
The Millard reactions reducing sugar reacting with alpha amino acid in cereals. Wheat at the crust impacts brown crust palatability and colour attractions on bread products. The glutein ,gladien and sulphur hydrly bond structure expansion as a result of gas entrapment which are release expanding the interterstatial matrixes. Wilcox had reported mechanical treatment to disrupt protein nature during kneading.
Steaming
Legumes food such as dry beans and dry soybean exhibit gelling properties below the isoelectric point allowing gel to hold water with the three dimensional network. This could be observed in soymilk and okpa, a local steamed meal from Bambara nut in Nigeria
Mixing
The water-soluble albumins and salt-soluble globulins of wheat grain comprise mainly metabolic and protective proteins that form the glutathione structure in dough. The proteins located in the wheat endosperm determine the characteristics of elasticity, extensibility, and gas-holding ability of the wheat dough. Particularly the low- and high-molecular-weight glutenin subunits, proteins, and the diverse group of gliadins.
Glycosylation events are characteristic on all secreted and membrane proteins, while phosphorylation is often linked to proteins related to enzyme metabolism, stress responses, and growth. Storage proteins of the cereal seeds are rich in amino acids like proline or glutamine.
Deamidation of glutamine-containing proteins into glutamic acid results in higher water-binding capacity. Polymer formation of storage proteins in wheat gluten by which sulfur-containing amino acids are bound to each other via disulfide bonds, so that a stabile chemical interaction is formed. These abilities of cereal and especially wheat grain proteins are important in the structure–function relationship analysis of wheat gluten for food products and also for nonfood
Pre cooking processes, such as washing and soaking, are performed to improved cooking quality.7 However, cooking, especially heat moisture treatment, can reduce protein digestibility of many cereals, such as rice8 wheat9 sorghum10 and millet11 due to secondary structure, hydrophobicity of hydrophobic protein (i.e., kafirins and zeins), and protein cross linkings (disulfide cross linking, hydrophobic cross linking, and isopeptide cross linking). In addition, Mujoo et al.,12 indicated that disulfide cross‐linkings are formed during rice flaking, and disulfide induced aggregation is susceptible to proteolysis. Kubota et al.,8 found that rice prolamin/protein body cannot be indigestive in cooked rice. However, Zhang et al.,13 suggested that the change in rice protein digestibility during cooking was associated with the species of protease.
Effect of pressure cooking
AIlo and Berghofer,14 found that some thermal instability amino acids including lysine, arginine, cysteine, methionine, and tryptophan are lost during oat powder extrusion. By contrast, no significant change was observed in these amino acids except cysteine. This finding may be because the intensity of rice cooking was milder than extrusion.12 However, slight reduction was observed in cysteine. It may be due to cooking induced oxidation in cysteine and or additional loss of cysteine during acid hydrolysis.13 In addition, decreases of serine and proline occurred after cooking, especially at high pressure cooking and microwave cooking; despite that microwave cooking is regarded as a preferable method that can prevent the excessive loss of nutrients in food matrix during cooking.13
The processing of rice using domestic microwave cooking showed no significant effects on protein content, despite the limited effect of rice pre cooking on protein digestibility. Disulfide bonds and hydrophobicity interactions were formed during the microwave cooking. However, cooking induced hydrophobicity interactions might not affect the protein digestibility. By contrast, disulfide bond cross linking during cooking decreased the protein digestibility observably. The heat induced formation of intermolecular disulfide linkages during cooking might stabilize and strengthen rice. In general, cooking had limited effects on rice amino acids structure .But Deng et al,15 assert that cooking pulses for three hours, the methionine, tyrosine and threonine content decreased. Pressure effect result in shear motion creating hydration of the protein because of the globulin matric stretching and arrangement of amino acid along the direction of the mass flow.
Effect of fermentation on protein
Fermentation has been used on pulses involving the raw seed16 flour17 or protein isolates.18 This may involve a solid-state batch fermentation or a submerged fermentation process involving protease-producing bacteria or fungi. Fermentation can improve the protein digestibility of pulses by reducing the levels of non-nutritive compounds that inhibit digestive enzymes (trypsin and chymotrypsin inhibitors) and promote protein crosslinking (phenolic and tannin compounds), as well as through the production of microbial proteases, which partially degrade and release some of the proteins from the matrix.19 Hemalatha et al.,20 reported that fermentation also improved mineral bioavailability, as microbial metabolism generates organic acids, which then form soluble complexes with mineral compounds preventing the formation of insoluble mineral-phytate complexes. In addition, fermentation can be employed with or without heating; thus it can bypass the loss of nutrients seen in cooking processes.
Gamma irradiation
However, the report of Liu et al.,21 that gamma irradiation affects proteins by causing conformation changes, oxidation of amino acids, formation of protein free radical sand rupturing of covalent bonds has been established.22 Also, chemical changes caused by gamma irradiation on protein include fragmentation, cross-linking aggregation and oxidation caused by oxygen radicals that are generated in water radiolysis.21 This effect could be related to the structure of each amino acid, simple amino acids increased upon irradiation, such as glycine, which undergo reductive deamination and de-carboxylation.22 In addition, aliphatic amino acids with increasing chain length provide additional C–H bonds for interaction with OH radicals which reduces the extent of oxidative deamination. Hemalatha et al.,20 reported that sulphur containing as well as aromatic amino acids are, in general, the most sensitive to gamma irradiation, while simple amino acids could be formed by destruction of other amino acids. However, there is a scarcity of information relating to the effects of processing with ionizing energy on most underutilized legumes.
Roasting/Cooking
Roasting: roasting of nut legumes result in native conformation of protein tertiary structures by cleavaging the hydrogen bonds, ionic or hydrophobic bonds. This produce thiol group or polar group which may be exposed creating cross linking, bonding effects, finally creating maillard and acrylamide products. There was progressive reduction in the level of protein from raw to cooked and from raw to roasting. Maillard reactions which lead to a type of browning are an interaction between the carbonyl group of a reducing sugar and the free amino group from an amino acid or protein. The resulting condensation product is converted by the Amadori rearrangement to the 1-deoxy-2-ketosyl compound. Browning then proceeds along complex pathways, the exact sequence being dependent on pH, temperature, concentration and the identity of the reactants. The crude protein results showed that the Maillard reactions were mild in the cooked groundnut seeds (cooked at 85-90°C for about 20 min) but the reactions were high in the roasted samples (roasting at 120-130°C for about 25 min), hence protein reduction in both cooked and roasted, with the colour of roasted groundnut normally brown.
During Immersion cooking of whey protein from soy product, B- lactogloblin and α lactoglublin are denatured.3
Steaming: steam could also be a unit processing in extrusion, sterilization, pasteurization. Protein for wheat legumes can be steamed and during this process, the tertiary and quaternary structures of protein are formed because of viscosity spread of the material resulting in exposure of more side chains.
Texturization (wet cooking) globuline proteins are unfolded during texturization like soya milk production, releasing it chain to neighborhood interactions. This involves solubilization before viscos solution is made. Protein hydrated before temperature and pressure affect the protein during a Belitze et al.3
Functionality of plant protein
The chemistry of hydrophilic R –group on the outside and hydrophobic inside the amino acid structure makes them behave like amino acid zwitterion structure. They behavior as electrolytes and exhibit chemical and physical properties. When they have high acidic group aspartic and glutamic acid. Its isoelectric PH will be low. If basic arginine and lysine high isoelectric point would be exhibited.
This isoelectric charge point confer certain degree of solubility hence ability to determine hydrolysis and other extractability. Other includes viscosity, emulsification, foaming ability and water absorption capacity. However these properties could be altered by salting.
Denaturation: The change in conformational structure from nativity of the protein is termed denaturation characterized by coiling, peptides and susceptible to hydrolysis by protein enzymes and other decrease functionality like solubility, catalytic actions and hormonal effects.
According to Wilcox protein denaturation includes
Gelling
Dry protein has ability to absorb water .few proteins can form gels capable of immobilization. These protein sources like from cereal , legumes and seed fruits have high asymmetric resulting from three dimensional hydrogen bond and this inter protein network could hold water. This cross link structure is well developed to hold water ionizable form aid in the gel formation. Above the isoelectric points the gel shrinks resulting in synerises.
Solubility
The conformational spatial arrangement of protein molecules makes them soluble either in water or organic solvent. This also helps in precipitation, purification and characterization of proteins. The solubility extends to fractional peptides like water affinity, viscosity, emulsifying ability, foaming ability and water retention ability. Protein solubility is improved by certain PH. The PH of 4-6 are isoelectric points and by increasing ionic value to 0.5 salting effect occurs resulting in precipitation Abnormal high salt content decreases solubility hence increases competition as a result of the added salt for water.
Water binding ability
Because of the site exposures or unexposed, protein material tends to bound water or absorb or retain water . The hydration ability is a function of polar sites, hydrogen bonding to phages ionic materials. Though altered by PH since some of these sites are polar and carboxyl grouped.
Matrix formation
The ability of certain protein from legumes to form structure on food such as foaming to hold moisture (protein film technology), carbohydrate, lipid. This rheological phenomenon of protein material come from complex interaction s which involves degree of solubility, shear force or shear stress, gelation, denaturation and coagulation .Heating altered the conformational structure of protein of cereal and legumes which do promote this.
Emulsifying capacity
This is based on its surface properties, it is important for plant protein for analogue studies like meat analogue from soybean. Emulsification is a heterogeneous phase having fat as a dispersed phase and continuous phase water with emulsifying agents being plant protein micelles, lowering the surface tension of water and oil interphase, maybe a desirable outcome from plant protein. Though, dependent on PH, ionic strength. The formation of film protection coating confers another desirable outcome. The ability of protein to maintain emulsion in food processing , such as pasteurization depends on heat of gelation and coagulating properties .This is good for milk, myaonaise and salad dressing and frozen desert.
Foaming ability/whipping
This property is good for confectionary making when plant protein like soybean, wheat etc are intended. Foam is a colloidal dispersion in which gas phase is in a liquid continues phase. During whipping for soymilk preparation, protein molecules unfold so that the polypeptides chains are aligned parallel to the surface. The conformational change result in loss of solubility resulting in coagulation .Also factors such as temperature tension PH surface area play significant effects.
Sensorial quality
The flavor, colour aroma resulting from Millard reaction contributes to those sensorial attributes. The millard reaction though complex produces amadori.
Gelation ability
Because of hydrogen bonding, hydration tendency of plant protein, they can form stable gel provided the chains are exposed and interchange . The ability of dry protein to absorb water equals to its weight of hydrated protein is term gelation capacity.
Plant proteins; outcome on foods and food systems
The nutritional value of plant protein is determined by its amino acid composition. A protein containing all of the essential amino acids in life and growth sustaining proportions is considered a complete protein and will have a high biological value. Plant proteins generally are not as high in biological value due to their deficiency in some of the essential amino acids. However, incomplete proteins can be supplemented with the missing essential amino acids. Hence supplementation technology.
Food proteins are responsible for texture, color, and flavor. Today they are extracted, modified, and incorporated into processed foods to impart specific functional properties. For example, proteins can function as buffering agents, emulsifiers, and fat mimetic in foods.
Certain proteins can also form gels and foams. Because proteins contain both hydrophilic and hydrophobic characteristics they can orient themselves at the oil–water interface and can stabilize emulsions, which is important for the stability of foods such as salad dressings, sauces and mayonnaise.
Foams are colloidal dispersion of gas in liquid. The protein orients itself at the air–water interface to trap air, similarly as emulsions. Foams are important in foods such as dessert toppings and ice creams. Proteins can also form a well-ordered protein matrix or a gel which then traps water, fat, and other food components. Food products like yogurt, tofu, and gelatin dessert rely on the gelation properties of proteins.
Food proteins can be powerful allergens for some people. Peanut, various tree nuts (such as walnuts, pecans, almonds, and cashews), soybean, wheat, milk, egg, crustacean, and fish proteins have been demonstrated to induce immunoglobulin E (IgE)-mediated food allergies.
There are also proteins, or amino acids that may react to form toxins. For example, acrylamide in fried potatoes is formed from the reaction of amino acid asparagine with reducing sugar heterocyclic hydrocarbons from fish proteins and wood charcoal.23 It is important to note that food processing can alter the nutritional value and functional properties of proteins, along with enzyme activity.
Some cereal proteins have unique properties which are very well suited for Specific industrial applications. For example, wheat gluten is suitable for making coatings/films and adhesions. In some industrial applications, proteins such as gelatin and casein are used in adhesives whereas soy proteins are used in paper.
Coatings
Cereal proteins in this natural form or in modified forms have great Potential for some industrial uses. Because of the excellent film forming properties, wheat glutens offer very good perspectives for use in coatings and films; hence Proteins of other cereals such as maize and sorghum need to be examined in details for this specific application. These types of coatings and films could have desirable effect in food sector and also in packing industry to adjust the barrier properties for gases, flavors and water vapor. Cereal proteins may provide a better barrier for oxygen than the synthetic polymers. This is of direct practical importance for packing of fresh products such as tomatoes.24–35
|
Foods |
Amino acids fractions |
Functionalities |
|
1.Cereals Wheat |
Albumin |
Water foaming, whipping, flour solubility, cereals enzymes initiation and synthesis, aids in fermentation |
|
Rice/wheat |
Globulins |
Salt-solution starch-protein matri formation, emulsification, gel consistency, flour solubility |
|
Sorghum /wheat |
Prolamins |
Aqueous ethanol Dough rheology, dough extensibility, extension of dough life, product quality, natural flavor. |
|
Rice/wheat |
Glutenins |
Dilute alkali or acid Elasticity and dough strength |
|
Sorghum /wheat |
Prolamins |
properties, resistance to shear, viscosity, bread loaf volume, controlled expansion, gas retention |
|
Maize |
Zein |
|
|
2.Lugums |
||
|
Soybean |
Throenin, Alanine , Leu, |
|
|
Groundnut |
Globulins, Tr, Leu Ph, Gly, Glu |
Salt-solution Starch-protein matri formation, emulsification, gel consistency, flour |
|
Flour |
||
|
Lentils |
leu, ly, thr.phya |
cyst |
|
Pigea peas |
Lysine, glutein, asp, meth, cyt |
|
Table 1 Major plant source protein
|
Amino acid |
Wheat |
Rice |
Maize |
Sorghum |
|
kg x 1000 |
kg x 1000 |
kg x 1000 |
kg x 1000 |
|
|
Lysine |
130 |
85 |
104 |
8 |
|
Met. & Cystine |
162 |
78 |
135 |
11 |
|
Threonine |
132 |
90 |
140 |
13 |
|
Isoleucine |
219 |
85 |
143 |
14 |
|
Tryptophan |
60 |
26 |
27 |
4 |
|
Valine |
209 |
127 |
189 |
16 |
|
Leucine |
313 |
189 |
484 |
47 |
|
Phen. & Tyr. |
404 |
199 |
339 |
30 |
Table 2 Annual global yield of essential amino acids from major cereals
|
Food |
Limited Amino Acid |
Complement |
|
Beans |
Methionine |
Grains, nuts, seeds |
|
Grains |
Lysine, threonine |
Legumes |
|
Nuts/seeds |
Lysine |
Legumes |
|
Vegetables |
Methionine |
Grains, nuts, seeds |
|
Corn |
Tryptophan, lysine |
Legumes |
Table 3 Protein complementation table
Proteins from plant are gaining recognition because of its unsaturation of amino acid and food potentials in human health. The chemistry of plant food is diverse, but the bed rock for supplementation, fortification, complementation and guide during processing for stability of its functional attachments. From the nature of proteins and heat labile characteristics of plant proteins, recognition must be given to for its protein quality, quantity and its rumen bioavailability in human nutrition as well as for safe product and storage technology.
None..
The author states there are no conflicts of interest.
None.

©2022 Ogori. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.