Journal of
eISSN: 2373-4310


Research Article Volume 13 Issue 2
1 Agricultural Engineering Research Institute, Agricultural Research Center, Egypt
2 National Institute of Laser Enhanced Science (NILES), Cairo University, Egypt
3 Agricultural Engineering Department, Agricultural Faculty, Cairo University, Egypt
4 Nile Research Institute, National Water Research Center (NWRC), Egypt
Correspondence: Abd El-Rahman Abd El-Raouf Ahmed, Agricultural Engineering Research Institute, Agricultural Research Center, Giza, Egypt
Received: April 13, 2023 | Published: May 25, 2023
Citation: El-Raouf Ahmed AERA, El-Sayed Hasan H, Suliman AERE et al. Effect of laser irradiation and Rhizobium on growth parameters of clover. J Nutr Health Food Eng. 2023;13(1):28-43 DOI: 10.15406/jnhfe.2023.13.00368
This study aim to study the effect different combinations of Laser or/and Rhizobium on growth parameters of clover plants. To carry out this study, irradiation seeds by laser radiation and seeds incubation by Rhizobium combination with laser exposed times 5, 10, and 15 minutes compared in untreated seeds were used. Growth plants were cut four times during growing season every 40-45 days, then growth parameters were measured. Obtained results were showed that using treat seeds by combination laser exposed 5 minutes and Rhizobium gave higher the growth parameters followed with 10 minutes and control. While, the expouser time of 15 minutes was less value. By using Laser exsedtimes or/and Rhizobium, the results were indicated that the growth parameters values of cover plants at irrigated with fresh water were higher than clover plants which irrigated with ground water. So, authors recommended to use irridation by Laser beam and / or incubation by Rhizobium of clover seeds or any different seeds crops in order to improve vegetation and productivity of crops.
Keywords: clover seeds, Rhizobium, inoculation, laser, growth parameters
Rhizobium inoculants for alfalfa (Medicago sativa L) production are typically delivered as coatings on pre-inoculated seeds or as peat-based powder formulations applied to the seed just prior to seeding. Although popular in the past, liquid formulations have a shorter shelf-life and are not a common choice for producers. Pre-inoculated seed is very convenient, requiring no additional operation at the farm level, but is susceptible to loss of viability of the applied rhizobia. Several studies performed on various crop plants showed that the effects of laser stimulation can depend on many factors: the wave-length, dose, exposure time, the species and cultivar of plant.1
Seed stimulation is performed most frequently with the use of low power lasers and relatively long exposure times, measured in seconds or minutes. Some researchers are of the opinion that seeds should be irradiated several times with small doses of radiation to eliminate the possibility of mutation.2 Higher doses influence the genetic material of the cell, leading to genetic changes of plant traits.3 One of the methods of seed refinement is irradiation; a divergent beam laser light provides energy to modify physiological and biochemical processes, causing an increase in the field’s performance to rise and yield crops.4 Bio-stimulation using He–Ne laser and laser diodes was applied to improve the physiological quality of certain crops.
It has been revealed that rhizobial inoculation of Phase olusvulgaris increased abundance of the bacterial and Rhizobiaceae communities especially at the flowering and the harvesting stage more than nitrogen fertilized controls.5
Compatibility between legumes and rhizobia can also be established at late stages of symbiosis, during the infection process and even in the nodule. Plants produce cystein-rich peptides that are secreted to control differentiation of rhizobia. In indeterminate nodules, which contain a persistent meristem, bacteroid differentiation includes end replication and cell expansion, leading to a developmental stage.6
Some of these markers of rhizobial identity can be crucial for the ecological outcome of the interaction, allowing plants to discriminate between different strains of a certain species with different symbiotic efficiencies. The partner selection theory proposes that plants have developed mechanisms to identify and select the best partners in a market scenario long before nodules are formed, and bacteria start to fix nitrogen.7 These fungi are responsible for the production of mycotoxins and these metabolites affect the seed quality, germination, viability, seedling vigor, growth of the root and coleoptile.8 However, information regarding the mycoflora on soybean seeds coupled with laser irradiation is scarce.
Khalifa et al.,9 indicated that the transformation of glutamate to proline may refer to the induction of proline biosynthesis in plants by increasing the thickness of the cell walls. Irradiation of seeds with laser light stimulates ATP production, and thus an increase in crops in the form of mass.10 In the conducted studies, the chlorophyll a fluorescence for triticale leaves was similar between the control and the plants grown from grains irradiated with laser. The long irradiation cycle most likely increased the consumption of metabolic products, which resulted in relatively in effcient use of energy resources during growth.11
The results suggest that in triticale leaves grown from grains irradiated with He-Ne laser, the excitation energy is transferred from the antennas to the reaction centers at the same level, compared to control. Contrary to light-exposed plants, for example, plants growing in the shade where a smaller number of electrons are transported to the intersystem chain are prone to slight photo inhibitory damage.12
To compare the effects of different laser wavelengths, exposure times, and low-power-intensity laser irradiation on maize seeds, seeds were exposed to He–Ne (632.8 nm) red laser, Nd:YAG second-harmonic-generation (532 nm) green laser, and diode (410 nm) blue laser. Four different exposure times (45, 65, 85, and 105 s) with different intensity (2 and 4 mW/cm2), for each laser were tested. Plant height was found comparatively high in blue laser light—211 cm at 85 s. The order of seed yield was blue (7003.4 kg/ha) > green (6667.8 kg/ha) > red (6568.01 t/ha) based on different exposure times of 85 s, 85 s, and 105 s, respectively, compared to the control of 6.9 kg/ha.13
Use of Ruby and He–Ne laser in agriculture for rapid seed germination and growth of the plants through activation of phyto chrome system. Several studies have demonstrated the potential, significant and positive impact of lasers on major crop plants. Recently, the use of lasers in agriculture is reviewed by Hernandez et al.1 The experiment aimed to determine the pre-sowing effect of irradiation time with laser He-Ne red light of triticale grains on selected morphological and physiological parameters of plants grown. In relation to the control, the elongation growth of seedlings was stimulated in grains irradiated with light for 3 hand inhibited for 24 h. The values of the fresh and dry mass of seedlings changed depending on the exposure time. Red light clearly stimulated the increase in the value of organ mass.14
However, many experimental studies suggest that exposure of dry and dormant seeds to He-Ne laser radiation triggers various other biological responses. The biological effect of a laser is not a single but a complex function of many factors. Changes in the electrochemical, biochemical, and optical properties of seeds are attributed to biological effects, mainly observed in the germination process and the analysis of the growth phases.15 Light intensity and wavelength are one of many factors of the primary driver of crop production. The sub-optimal values of light intensity can limit crop yield and reduce product quality. Therefore, studies on the use of laser irradiation with the appropriate wavelength are a chance to improve the quality and quantity of crops.16
The He-Ne laser plays a positive role in stimulating plant growth and metabolism and protects against biotic and abiotic damage caused by various adverse environmental factors.17 It also increases tolerance, mainly by improving the rate of seed germination, as well as stimulating elongation growth of plants and their biomass.4 The effects of pre-sowing bio-stimulation on plant crops showed that the best results of this treatment are obtained for plant vegetables, rather than for cereals and root crops. After applying radiation laser to tomatoes and cucumbers, a significant increase in yield was found, which accelerated the ripening and produced better fruit quality.18
Morphological changes in the plants grown from the seeds treated by He–Ne laser light of 50 mW, were observed; they were taller and thicker than plants from seeds without irradiation (control), as reported by Sreckovic et al.,19 Abu Elsaoud & Tuleukhanov20 whose found that seeds pretreated with a laser (I = 5mW/mm2) significantly increased the germination and growth parameters of four wheat varieties after seven days of treatment. Muthusamy et al.,21 applied treatment of low-intensity laser light at different irradiation doses on eggplant seeds and observed changes in later growth stage and yield; the best results were found radiation doses of 25 and 30 mJ/cm2. The highest value of stimulation in the height of the plants was obtained at t = 20 s, with increased growth (stem diameter, root length, and height of plants) for the exposure times of 5, 10, and 20s.22
Similarly, the effects of the spectral impact of the laser on seed performance were reported.23 laser irradiation induces a change in the plant development and makes the cell divide faster, which results in faster initial rate of growth and improvement. Therefore, the significant effect of laser light on growth and yield parameters in the present study could have been due to an increase in cell division.
The pre-sowing seeds irradiated with blue (410 nm), red (632.8 nm), and green (532 nm) laser lights had effects on the seed yield and the harvesting index characteristics of the plant. The results of seed yield indicated that the blue laser light had a better influence on maize seed than without the light condition (the control experiment).
Abdullateef & Osman24 Laser stimulation mechanisms in some physiological states of plants is synergism between the monochromatic and polarized laser light beam and the photoreceptors of light. The irradiation effect of the maize sowing seeds stimulates an increase in seed yield. In the bio-stimulation of plants in their different phases of development, three classes of photoreceptors were discovered: phyto chromes, photo tropins, and crypto chromes. These photoreceptors absorbed different wavelengths, i.e., 600–750, 320–500, and 500–630 nm, respectively.25 The vegetative growth of soybean seedlings was significantly higher after laser treatment; the magnitude ofthe stimulation for seedling growth was a function of the time exposed to laser treatment.26 Rassam27 stated that laser treatment could be used to control the fungal infection in wheat seeds andimprove their growth and development.
Many authors prove that the success of laser stimulation is dependent on wavelength, irradiation time and irradiation dose.28 Generally, seed stimulation is done with the use of small doses of laser radiation. Lower doses of laser activate plants, resulting in increasing bio energetical potential of the cells and higher activation of their biochemical and physiological processes.29
The seeds of brinjal (Solanum melongena L.) var. Mattu Gulla were irradiated with single exposure of He–Ne laser at different doses of 5–40 J cm)2 and germinated aseptically. A significant enhancement in growth characters were noted with respect to length, fresh and dry weight of shoots and roots, wereobservedintheirradiatedseedscomparedwiththeun-irradiatedcontrols. In conclusion, the results were demonstrated that low dose (5–30 J cm)2) of He–Ne laser irradiationenhancedthegerminationprocessandalteredgrowth,bypositively influencing physiological and biochemical parameters of the brinjal seedlings compared with un-irradiated control under invitro conditions.30
Dziwulskaand31 have reported that the stimulation by the laser energy is a physical phenomenon which includes absorption and radiant energy storage by cells and tissues of plant systems. The seeds also have the same phenomenon; first they absorb the laser energy and then convert it into chemical energy which will be utilized for germination and growth. The growth characters, viz., length, fresh and dry weight of shoots and roots, number of leaves and roots were higher at lower doses of laser irradiation was found to be decreased at higher doses. Similarly, the dry weight of roots, number of leaves and roots were also significantly higher in lower doses of laser radiation compared with un-irradiated control. Among the growth characteristics, length, fresh weight of shoot, dry weight of root, number of leaves and roots were higher at 25 J cm)2 whereas the fresh weight of root and dry weight of shoot washigher at 20 J cm)2 laser irradiation. The laser irradiation induced significant improvement in seed germination and subsequent growth of seedlings.15
Govil et al.,32 reported the maximum increase in shoot and root length, dry weight of the seedlings from laser irradiated (30 min) green gram seeds and enhanced emergence, early flowering and maturity, plant height due to the presowing He–Ne laser treatment than the control plants off ababean.
Osman et al.,33 studied effect of He-Ne laser at different exposure periods (5, 10 and 20 min.) with power density of 95 mW/cm on two plants (Fe on iculumvulgare Mill and Coriandrumsativum L.). In most cases, the highest plant heights and number of branches per plant were obtained from the treatment of 20 min exposure. On the other hand, Aguilar et al.,34 reported that laser irradiation could cause enhancement of enzyme activities. A positive influence of seed irradiation on the processes of growth and development of crop plants, mainly cereals, legumes and certain vegetables. Pre-sowing laser stimulation can cause an improvement of germination and emergence, an increase of yields or a shortening of the period of vegetation, and even enhance the resistance of plants to environmental stresses.35
Seed stimulation with all of the doses of laser light cause dane longation of the radicle. 1- and 5-time irradiation with laser of 6 mW cm–2 power and 5-time irradiation with laser of 8mWcm–2 power cause dan increase of seedling radicle length relative to the control. Whereas,in seed stimulation with laser of 8mWcm–2 power cause danelongation of the radicle.These results confirm the research of other authors,indicating that laser radiation cause sanelongation of roots of crop plants. Drozd36 Reduction in time for 50% germination for laser treatment group (20, 25 and 30 J cm)2) was found to be significant (P < 0.001) compared with un-irradiated control group. Influence of different laser doses on growth parameters of in vitro raised brinjal seedling is indicated in Increased shoot length.37
El-Tobgy et al.,38 confirmed that the red light laser can induce GA3 which promotes the complex cycle of GA formation, while the polychromatic light (sunlight) cannot induce this effect. Chinese pine seeds irradiated by He-Ne laser led to an enhancement for seed vigor, germination rapidness, root system expansion and fresh seedling mass.39 Podlesna et al.,40 studied the effect of pre-sowing laser radiation of pea seeds on seed biochemical processes, germination rate, seedling emergence, growth rate, and yield. The authors showed that the properly selected power and time of laser exposure can repair the DNA structure in plant cells and shorten the time of cell regeneration.
Objectives of this study are : This study aim to study the effect different combinations of Laser or/and Rhizobium on growth parameters of clover plants., and using different irradiation seeds by laser radiation and seeds incubation by Rhizobium combination with laser exposed times compared in untreated seeds to select the best treatment.
Clover seed samples
Clover Seeds (Trifolium Alexandrinum L.) (Giza 6 Variety) samples were obtained from the Agricultural Research Center, Field Crops Research Institute (FCRI) Giza, Egypt. Clover seeds were direct seeding in pots for a full growing in Agricultural Research Center, Land, Water and Environmental Research Institute, during the 2020/2021season., under a greenhouse without organic manure application.
Three samples of clover seeds were exposed by laser radiation with wavelength 632.8 nm)with three times (5, 10 and 15 minutes) and three samples seeds were incubated by Rhizobia with of 5, 10 and 15 minutes of laser radiation time) and one sample without any treatment (control) and other sample with Rhizobia only as control. The total of ten samples of clover seeds after irradiating by two treatments were cultivated. Clover Egyptian cultivar was evaluated in two different conditions, irrigated in Nile water and groundwater, the seeds were inoculated with Rhizobium meliloti before seeding and other seeds were exposed by laser and combination with Rhizobium before seeding too. The experimental soil is clay- loamy and a randomized complete block with four replicates. Figure 1: Schematic diagram of the experiment.
Methods
Seeds clover exposure by laser beam for seeds irradiation were carried out in the Laboratory of Laser Applications in Agricultural Engineering at the National Institute of Laser Enhanced Science (NILES), Cairo University, for quality evaluation of clover plants during growing period (after each cutting) by seeds treatments by He-Ne Laser 632.8 nm irradiation and Rhizobia incubation treatments.
Description of the apparatus: The optoelectronic apparatus was manufactured and developed to provide irradiation clover seeds samples with different doses. The Opto – electronic apparatus is shown in Figure 2, consists of the following main parts:
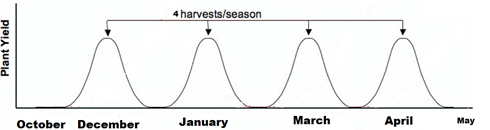
Figure 2 Model of pesticide inhibition of symbiotic recruitment resulting in reduced harvest yields to host plants by 4–6 weeks after treatment.
Stand holder, it was designed and fabricated of iron as a shape square with length 45 mm and total length 900 mm , it contains seven holes to adjust the height and direction of laser and beam expander to insure a good aliment of the laser beam on measurement area. The stand holder was fixed as a vertical on an optical bench by two screw bolts.
Beam expander: The reverse Galilean telescope design provides a certain angular magnification, called the Expander Power. The beam diameter is first increased in size by its power. The expander was used in the optical unit in front of Helium-Neon laser. Each laser beam size 1 mm expanded in order to cover area 20X30 mm2. The specifications of beam expander were as follows:
- Dimensions of the expander with length of 280mm, maximum and minimum diameters of 110 and 50 mm.
- Source of manufacture: Egypt, made in hard Aluminum, electrostatic black paint.
- Beam expansion power: 50X
- Lenses: 1 st Diverging lens: F = 20 mm; Dia.= 15 mm;
2nd Diverging lens: F = 2.2 mm; Dia.= 10 mm;
Collimating lens: F = 240 mm; Dia. = 100 mm (F is the focal lens)
Optical bench: The optical bench was fabricated from stainless steel. The dimensions of this bench were 880, 580 and 45 mm in length, width and height, respectively, with screw holes 6 mm diameter and the distance between them was 50 mm. Bench was manufactured in USA sitting on the second frame.
Irradiation treatment by laser
Clover seeds were exposure to He-Ne laser irradiation (wavelength 632.8 nm, power density 8 mW) treatments, i. e. control, periods 5, 10 and 15 min exposure. Laser applications have been carried out at the National Institute of Laser Enhanced Sciences (NILES), Laser Technology Centre, Cairo Univ., Giza, Egypt. Laser light emitted from the standard device is generally characterized in terms of power (in units of watts and milliwatts). The power levels vary from laser type to another.41
The energy (in joules) is defined as the power × time interval during which it is emitted, i.e. energy (Joules) = power (W) × time (sec). The laser beam has a spherical shape of the particles, with an average other parameters which are especially important.42,43
The power density or irradiance is then defined as the power applied per unit area of target tissue. Fluence is defined as the energy applied to an area of target tissue, i.e.
Fluence = power density (W/cm2) x time (sec)
The experiment uses of clover seeds cultivation by treated seeds with Laser or/ and Rhizobium, which mean sixty four pots and it is illustrated in (Figure 1).
Clover seeds treated in nodules
1-Rhizbium rate: A two-node bag (200 g) is sufficient for seeds of one acre (feddan) for old lands.
2-Gummy solution: Prepare a sugar solution (4 tablespoons of sugar dissolved in 2 cups of water).
3- Mixed seeds by solution and Rhizobium: A large plastic mattress is placed in a shady place, then the seeds to be inoculated are placed on it, then all of them are mixed with the sugar solution, where all the seeds are moistened and then add the two nodules bag to the seeds and mixing well to ensure distribute all the contents of the bag to the seed.
Pre-treatment seeds
To evaluate the effects of Rhizobium incubation and Laser radiation on growth and biomass production of clover cultivar under 4 levels of laser irradiation namely: 0 as control, 5, 10, and 15 minutes using He–Ne laser (632.8 nm), with a power density of 8mW, also and 4 levels of Rhizobium with laser irradiation namely: 0 with Rhizobium only as control, 5 minutes with Rhizobium, 10 minutes with Rhizobium, and 15 minutes with Rhizobium minutes under greenhouse experiment was designed.
Seeds cultivation
Pre-germinated seeds were sown in 5 kg plastic pots filled with fertilized soil of 20, 25 and 10 N, P, K mg kg-1, respectively. Also, 15N-labelled NH4NO3 was uniformly applied within each treatment plot. Then, fifty seeds of each cultivar were sown in each potin 2.5 cm deep using a hand. on October 2020, thinned to twenty seedlings at the two leaf stage, and harvested at 10% flowering stage on 3 April, 2021. Treatment pots were 30 cm diameter and 30 cm height filled with soil and two a plastic reservoir where hanged over the top of the pots with a valve for continuous filling the tank with waters. (One filled with Nile water and the second groundwater).
Water irrigation types sampling
The water needs of Egyptian clover differ in the days of the plant age, where that the needs of clover decrease in the winter months and gradually increase from the beginning of March until the end of the season in May. Two types of irrigation water: Nile water, and ground water were used.
Soil analysis
Soil samples were collected for seeding and analyzed by Central Lablatotries, National center of Water Researches. Soil Physicochemical Analysis (Tables 1&2), analyzed according to Qian PY.44 Soil traits such as salinity, pH, calcium, magnesium, potassium, sulfate ion, bicarbonate, chlorine, and the percentage of sand, silt and clay of the study area were determined.
|
Items |
Value |
|
Textural class (%) |
Particle size distribution |
|
Sand % |
25.5 |
|
Silt % |
35.8 |
|
Clay % |
38.7 |
|
Texture grade |
Clay loam |
|
CaCO3 (%) |
2.28 |
|
Saturation percent (S.P %) |
42.1 |
|
Soil properties |
|
|
pH |
7.79 |
|
E.C (dS m-1) |
1.12 |
|
Cations (meg/l) |
Soluble cations (meq L-1): |
|
Ca++ |
4.22 |
|
Mg++ |
2.88 |
|
Na+ |
3 |
|
K+ |
1.19 |
|
Anions (meg/l) |
Soluble anions (meq L-1): |
|
CO=3 |
0 |
|
HCO-3 |
3.28 |
|
Cl- |
4.33 |
|
SO=4 |
3.68 |
|
Organic matter ( % ) |
0.82 |
|
Total soluble N (mg kg-1) |
56.3 |
|
Available-P (mg kg-1) |
7.5 |
|
Available-K (mg kg-1) |
372.3 |
|
DTPA-extractable (mg kg-1): |
|
|
Fe |
4.78 |
|
Mn |
3.21 |
|
Zn |
1.2 |
|
Cu |
0.59 |
Table 1 Soil physicochemical properties of the experimental
|
|
Fresh water |
Groundwater |
||
|
Physicochemical parameters |
||||
|
pH |
7.88 |
7.94 |
||
|
Carbonate |
CO₃ |
mg/kg |
0 |
0 |
|
Bi Carbonate |
HCO₃ |
mg/kg |
160 |
338 |
|
Total Alkalinity |
mg/kg |
160 |
338 |
|
|
Electrical Conductivity |
EC |
mmhos/cm |
0.33 |
1.135 |
|
Total Dissolved Solids |
TDS |
mg/kg |
211 |
727 |
|
Ammonia |
NH₃ |
mg/kg |
<0.01 |
0.3 |
|
Biological Oxygen Demand |
BOD |
mg/kg |
4 |
1 |
|
Chemical Oxygen Demand |
COD |
mg/kg |
8 |
2 |
|
Major cations |
||||
|
Calcium |
Ca |
mg/kg |
25.59 |
73.78 |
|
Potasium |
K |
mg/kg |
3.9 |
11 |
|
Magnesium |
Mg |
mg/kg |
13.31 |
28.68 |
|
Sodium |
Na |
mg/kg |
19 |
115 |
|
Major anions |
||||
|
Flouride |
F |
mg/kg |
0.33 |
0.35 |
|
Chloride |
Cl |
mg/kg |
12.8 |
76.3 |
|
Nitrite |
NO₂ |
mg/kg |
<0.2 |
<0.2 |
|
Nitrate |
NO₃ |
mg/kg |
<0.2 |
<0.2 |
|
Phosphate |
PO₄ |
mg/kg |
<0.2 |
<0.2 |
|
Sulfate |
SO₄ |
mg/kg |
15.6 |
169.9 |
|
Trace metal |
||||
|
Aluminum |
Al |
mg/kg |
0.071 |
0.034 |
|
Antimony |
Sb |
mg/kg |
<0.009 |
<0.009 |
|
Arsenic |
As |
mg/kg |
<0.006 |
<0.006 |
|
Barium |
Ba |
mg/kg |
0.052 |
0.125 |
|
Cadmium |
Cd |
mg/kg |
<0.002 |
<0.002 |
|
Cromium |
Cr |
mg/kg |
<0.002 |
0.028 |
|
Cobalt |
Co |
mg/kg |
<0.003 |
0.024 |
|
Copper |
Cu |
mg/kg |
0.038 |
0.059 |
|
Iron |
Fe |
mg/kg |
0.032 |
0.359 |
|
Lead |
Pb |
mg/kg |
<0.007 |
0.017 |
|
Manganese |
Mn |
mg/kg |
0.03 |
0.632 |
|
Nickel |
Ni |
mg/kg |
0.007 |
0.023 |
|
Selenium |
Se |
mg/kg |
<0.007 |
<0.007 |
|
Tin |
Sn |
mg/kg |
<0.006 |
<0.006 |
|
Vanadium |
V |
mg/kg |
0.03 |
<0.001 |
|
Zinc |
Zn |
mg/kg |
0.031 |
0.071 |
|
Microbiological parameters |
||||
|
Total Coliform |
CFU/100ml |
500 |
0 |
|
|
Fecal Coliform |
CFU/100ml |
300 |
0 |
|
Table 2 Water physicochemical properties of the experimental
Plant measurements
Twenty plants per treatment were used to assess the plant height and number of nodules at 4 growing vegetative stages (67, 123, 154 and 174 days after sowing: DAS). Shoot and nodules samples were dried at 70°C for 48h, and then shoot dry weight of each plant was recorded using a digital balance.
Fresh weight (FW)
FW is the fresh weight was using a Balance device with an accuracy of two decimal numbers (0.00 mg), to weight fresh shoots and roots of clover plants.
Dry weight
At final harvest, the forage was cut, rinsed briefly in deo inized water to remove on surface accumulated salts and dust, weighted and dried in an oven of 60°C heat for 48 hours, to determine the dry matter content.
Clover shoots dry weight
The shoots of five plants were harvested randomly from each of the 15N micro plots. Shoots were oven-dried (65°C) for 48 h, ground to pass through a 0.4-mm sieve and subsequently pulverized in a rotating ball-bearing mill.
Subsamples (1.0 ± 0.15 mg) were analyzed for clover shoots% 15N using a continuous flow, isotope ratio mass spectrometer interfaced with a Robo Prep Sample Converter (Europa Scientific, Crewe, UK).
Clover roots dry weight
The root system of five plants was dug out of each plot using a shovel. The roots were rinsed with water, blotted dry. Roots were oven-dried (65°C) for 48 h, ground to pass through a 0.4-mm sieve and subsequently pulverized in a rotating ball-bearing mill.
Subsamples (1.0 ± 0.15 mg) were analysed for clover shoots% 15N using a continuous flow, isotope ratio mass spectrometer interfaced with a Robo Prep Sample Converter (Europa Scientific, Crewe, UK).
Root nodule number
Root mass was washed with tap water and measured duration of the life cycle of root nodules –recorded on the basis of internal color (days)45 roots to the number of root nodules ratio was calculated (g roots/number root nodules) - dry root mass, dried at 60oC was divided to the number of root nodules.
Clover cut / mash (Harvesting)
Clover is harvested approximately every 45 days, which yields an average of four harvests per growing season. Clover varieties are characterized by the multiple periods of vegetative growth and thus the multiplicity of weeds, as these varieties are given between 4-5 plants.
A multi cut of clover was harvested in the last week of May (2021) and four cuts were harvested in the production years (2020 and 2021): one in mid-December, second in the last January, the third in a mid- March and the fourth in a last April. To obtain the highest yield, it is preferable to mulch when the height of the plant reaches from 40 to 50 cm because it is associated with the increase in the yield. The plants were cut 15 cm from the soil surface. Removed biomass was bagged, air-dried and weighed.
Statistical analysis
Correlation and path (standardized partial regression coefficient) coefficients were computed by the software SPSS (version 15) and indirect effects of the Excel spreadsheet program. The result of path analysis was displayed diagrammatically as a path diagram, for each condition.
Effect of treatments (Laser or/ and Rhizobium) on morphological clover plants in irrigation with fresh water and ground water
Effect of treatments on plants height
For using fresh water, the results were indicated that plant heights of clover plants were increased of (29.5, 31.3, 32, 33 and 33.5 cm), (33.5, 35.3, 36.7, 38.8 and 41 cm), (45, 50, 51.5, 52.5, and 55.5 cm) , and (55, 55.5, 56, 58.8 and 60 cm) for (N2, Rhizobia, N2+Laser, N2+Rhizobia, and Rhizobia+Laser) for first, second, third and fourth cutting of clover plants, respectively (Figure 3).
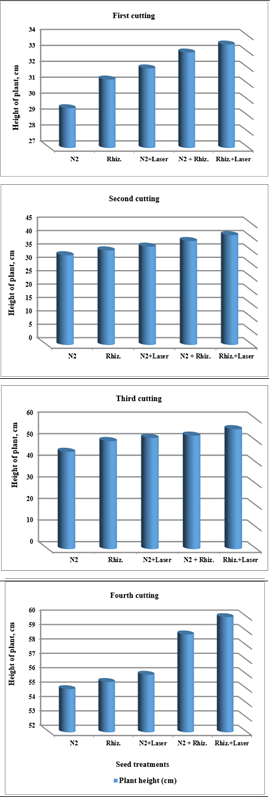
Figure 3 Effect seed treatments on height of plant in clover cultivation at irrigation with fresh water.
For using ground water, the results were indicated that plant heights of clover plants were increased of (21.5, 24.5, 27.7, 28.8 and 31 cm), (32, 32.3, 33.7, 36.3 and 38.4 cm), (41, 44, 48, 50 and 51.5 cm) , and (51.5, 52.5, 54, 55, and 56 cm) for (N2, Rhizobia, N2+Laser, N2+Rhizobia, and Rhizobia+Laser) for first, second, third and fourth cutting of clover plants, respectively (Figure 4).
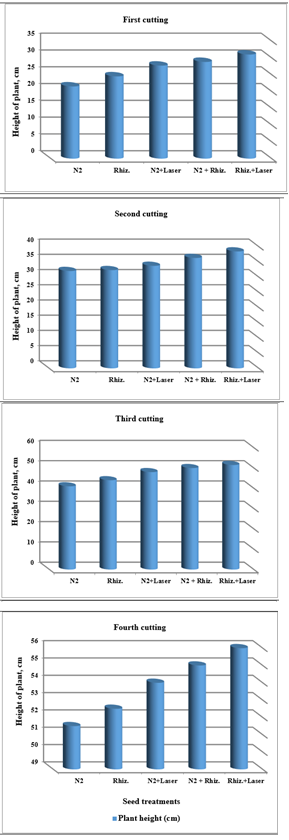
Figure 4 Effect seed treatments on height of plant in clover cultivation at irrigation with ground water.
Inoculation with diazotrophic bacteria like Rhizobium enhanced the plant growth as a result of their ability to fix atmospheric nitrogen46 Basu et al.,47 reported that Rhizobium inoculation was more effective for increasing the growth traits, yield and oil content of Arachishypogaea.
Deshwal et al.,48 concluded that the use of rhizobia inoculant enhanced plant growth in Mucuna plant. Not only Growth parameters were significantly influenced by biological and chemical fertilization but also it depended on growth stages.
Therefore, the most important finding is that Rhizobium inoculation improve growth parameters compared to nitrogen fertilization only at Advanced growth stage for “plant hight”; “Plant leaf area” and “Shoot dry weight”).
Uninoculated plants were not able to form efficient nodules as those that had been inoculated at all plant stages. Nitrogen fertilization has no significant effect on nodulation (number and weight) at different growth stages. Conversely, Rhizobium inoculated plants showed a significant high mean number and weight of nodules per plant. Rajendran et al.49
The reduction in plumule length as a result of drought stress is due to plant breathing disorders in initial germination and photosynthesis in bifoliate stages. These results are in accordance with the findings of Hamidi.50 Data indicated that the ratio of radicle to plumule length was increased by increasing drought stress. The ratio of radicle length to plumule length is a flexibility trait which it was increased as the result of drought stress.51
German et al.,52 observed that associative nitrogen fixing bacteria and phosphate solubilizing bacteria play a positive role in promoting the increase of root length, plant size and root surface area, as well as the assimilation of water and nutrient, and help to strengthen the respiration in root.
According to Chen et al., for example, He-Ne laser pretreatment can improve the inner energy of seeds, lead to an enhancement of cotyledon enzymes and speed up the metabolism of the cell, significantly increased the cycles of cell division (mitosis) which results in an increase in the length of the plant organs during the early growth.
Effect of treatments on shoot numbers of clover plants
For using fresh water, the results were indicated that the shoot numbers of clover plants were increased of (7, 8, 9, 10 and 12), (10, 11, 12, 13 and 14), (14, 15, 16, 17 and 18) , and (18, 19, 20, 21 and 22) for (N2, Rhizobia, N2+Laser, N2+Rhizobia, and Rhizobia+Laser) for first, second, third and fourth cutting of clover plants, respectively (Figure 5).
Figure 5 Effect seed treatments on shoot numbers of plant in clover cultivation at irrigation with fresh water.
For using ground water, the results were indicated that the shoot numbers of clover plants were increased of (5, 6, 7, 8 and 9), (7, 8, 9, 10 and 12), (12, 13, 14, 15 and 16), and (16, 17, 18, 19 and 20) for (N2, Rhizobia, N2+Laser, N2+Rhizobia, and Rhizobia+Laser) for first, second, third and fourth cutting of clover plants, respectively (Figure 6).
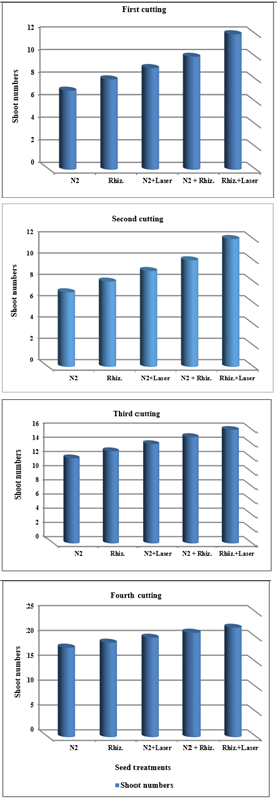
Figure 6 Effect seed treatments on shoot numbers of plant in clover cultivation at irrigation with ground water.
At each time point measured after each cutting clover plants, all parameters of SNF efficiency, including the number of shoots, the plant heights, the number of nodules, nitrogenase activity, and plant yields, were consistently highest for laser and the Rhiz-positive control group and lowest for laser or and the _Rhizunin oculated negative control group. The above parameters were measured at 6 weeks after cutting of clover plants. Results are mean values for four replicate populations of water used for irrigation of crops at fresh water and groundwater.
In the present study, we have noted the significant improvement in both amylases and protease activity in shoot and root of laserirradiatedseedlings.Thea-amylaseactivitywasincreasedovercontrol in both shoot and root and maximum activity was observed at 25Jcm)2. The b-amylase activity also shows similar trends with a-amylase activity and the maximum activity was noted at 20 J cm)2 in shoots and roots. The protease activity also showed increasing trends over control. Have demonstrated increase in amylases, transaminase and proteinases from the cotyledon of Isatisindi gotica treated with He–Ne laser and microwave irradiation.
Examining the effect of seed irradiation time on the elongation growth of triticale seedlings (expressed in cm units and as % of control by using the IP index) revealed that, the longest seedlings were found among grains irradiated for 3 h. In these cases, the He-Ne laser had a positive effect on the number of shoots, elongation growth, and dry mass.32
In general, the red light of the laser stimulated the morphology and physiology of seedlings and plants, although, for some features, long exposure to red light caused a slight reduction effect. The positive effect of laser radiation on plant growth and development can be caused by the ‘excitement’ of the bio-energetic structure by the formation of cells with excess energy and an increase in bio-energy levels in organisms.53
The growth characters, viz., length, fresh and dry weight of shoots and roots, number of leaves and roots were higher at lower doses of laser irradiation was found to be decreased at higher doses. Similarly, the dry weight of roots, number of leaves and roots were also significantly higher in lower doses of laser radiation compared with un-irradiated control.
Effect of treatments on nodules number confirmation of clover plants
For using fresh water, the results were indicated that the nodules number conformation of clover root plants were increased of (50, 55, 65, 72 and 75) , (60, 67, 72, 74 and 78) , (65, 73, 80, 91 and 98) , and (91, 99, 115, 121 and 131) for (N2, Rhizobia, N2+Laser, N2+Rhizobia, and Rhizobia + Laser) for first, second, third and fourth cutting of clover plants, respectively (Figure 7).
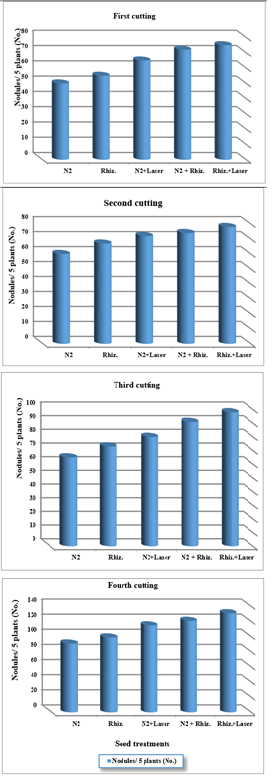
Figure 7 Effect seed treatments on nodules per five plants in clover cultivation at irrigation with fresh water.
For using ground water, the results were indicated that the nodules number conformation of clover root plants were increased of (30, 40, 45, 55 and 65) , (48, 55, 60, 65 and 78) , (56, 67, 73, 84 and 90) , and (86, 90, 94, 98 and 105) for (N2, Rhizobia, N2+Laser, N2+Rhizobia, and Rhizobia +Laser) for first, second, third and fourth cutting of clover plants, respectively (Figure 8).
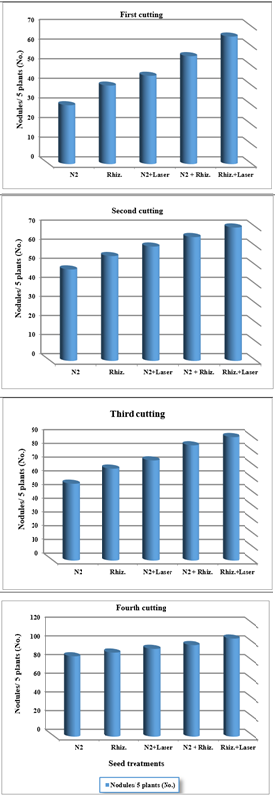
Figure 8 Effect seed treatments on nodules per five plants in clover cultivation at irrigation with ground water.
Furthermore, both rhizobia and alfalfa nodules are most abundant in the 30- to 60-cm soil layer, possibly because nodules in this region are the most actively fixing.
In the present study, plant response to the different inoculant formulations differed among treated seeds by rhizobiz or and laser treatments and untreated seeds.
Pii et al.,40 reported that Rhizobium inoculation increased nodule number on Medicagospp. Yadav & Verma46 stated that the dual combinations of Rhizobium leguminosarum either with Pseudomo nasaeruginosa, Bacillus megaterium and Azobacterchroo coccum, significantly enthused nodules number and dry weight of nodules. Granular inoculants placed below the seed encourage nodulation on lateral roots in both pulse and forage legume crops rather than the clusters of nodules in the crown of the plant that is often observed with seed applied inoculants.54
In fact, the non-treated plants exhibited fewer nodules numbers throughout the course of experiments, the same results obtained from the un-inoculated Rhiz-negative control plants. These data indicate that treatment with rhizobia and laser is sufficient to significantly nodule formation. Because nodule formation is necessary for symbiosis and the site of SNF.
It was noticed in the nodules number conformation of clover root plants were increased by using fresh water comparing in ground water in same treatments. Also, nodules numbers were gradually increased after first, second, third and fourth cutting of clover plants. There is a significant difference was shown between the un-inoculated plants (nitrogen fertilizer) and the Rhizobium or and Laser of inoculated plants concerning the nodule number per plant at all plant cutting stages (Four cutting harvesting).55
In fact, the greatest usually apprehensive modes of action of PGPR were phytohormone production, which improves plant development and growth.56 Possible coaction of Rhizobium bacteria inoculum and PGPR hosted in Goubell at soil could increase nodule number and nodule dry weight. Selecting efficient Rhizobium strains under greenhouse and field conditions for lentil, pigeon pea, clover and Chickpea increase nodule dry weight and shoot dry weight.57
Uninoculated plants were not able to form efficient nodules as those that had been inoculated at all plant stages. Nitrogen fertilization has no significant effect on nodulation (number and weight) at different growth stages. Conversely, Rhizobium inoculated plants showed a high mean nodule number per plant and a significant high mean nodule weight per plant.49
Effect of treatments on shoots and roots dry weight per twenty plants
Effect of treatments on shoots dry weight per twenty plants: For using fresh water, the results were indicated that the shoot dry weight (SDW) of clover plants were increased of (9.92, 10.40, 11.00, 12.19 and 14.92 g) , (9.99, 12.23, 14.60, 15.55 and 16.88 g) , (11.23, 13.55, 15.65, 17.89 and 19.34 g) , and (13.55, 14.61, 16.89, 18.99 and 22.33 g) for ( N2, Rhizobia, N2+Laser, N2+Rhizobia, and Rhizobia+Laser) for first, second, third and fourth cutting of clover plants, respectively (Figure 9).
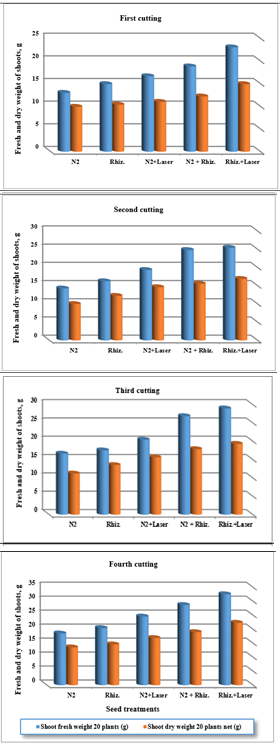
Figure 9 Effect seed treatments on fresh and dry weight shoots of plant in clover cultivation at irrigation with fresh water.
While, the results were indicated that the shoot dry weight (SDW)of clover plants were increased of (9.22, 9.56, 10.63, 11.23 and 12.54 g) , (9.56, 9.89, 11.59, 13.56 and 14.25 g) , (10.11, 10.55, 11.85, 13.99 and 15.44 g) , and (12.67, 13.55, 15.34, 17.35 and 19.25 g) for (N2, Rhizobia, N2+Laser, N2+Rhizobia, and Rhizobia+Laser) for first, second, third and fourth cutting of clover plants, respectively, for shoot dry weight (SDW) at irrigation by ground water (Figure 10).
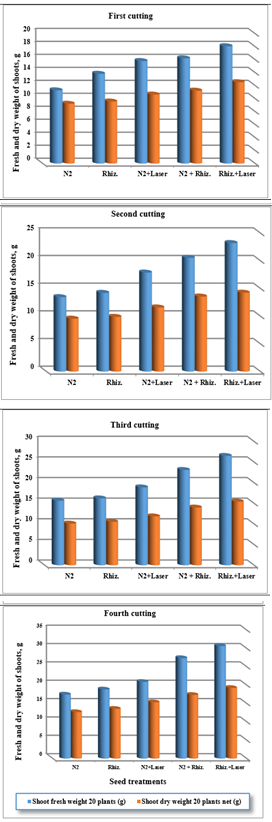
Figure 10 Effect seed treatments on fresh and dry weight shoots of plant in clover cultivation at irrigation with ground water.
Alfalfa seedlings were found obviously promoted after being inoculated with LW135 or RSN219. Shoot height, root length, root volume, individual number of leaves per plant, biomass and P uptake percentage of the two varieties were found remarkably increased than control group (CK1). This shows that after the inoculation of phosphate solubilizing Rhizobium and Klebsiellabacteria the productivity and growth of alfalfa were efficiently promoted in soil that is deficient in nitrogen and available phosphorus.55
As plant height and shoots number, shoot dry weight (SDW) for Rhizobium or and Laser inoculated plants was significantly higher than the uninoculated check (nitrogen fertilized and or laser).Shoot dry mass, and N uptake in above-ground tissue were measured for first, second, third, and fourth cutting at the irrigation of both fresh water and groundwater during season cut. Shoot dry mass production was very low in untreated seeds compared to treated seeds by rhizobia and laser.
In the pot experiment, Rhizobium inoculation and all nitrogen were increased the height of plant. None of the treatments had any effect on the number of leaves/plant. Also, all nitrogen were increased shoot fresh weight with or without Rhizobium. Rhizobium significantly increased the fresh and dry weight of shoot and root. Rhizobium inoculation also increased the number of nodules per plant. Nitrogen, with or without Rhizobium, significantly increased the number of nodules per plant up to 80 kg N/ha.58
The presence of a few weak nodules on the roots of uninoculated alfalfa plants indicates the presence of indigenous Rhizobium melilotistrains in Shambat soil, probably due to earlier inoculants applied in this area. The effect of Rhizobium in improving plant height, shoot fresh and dry weight, root fresh and dry weight, number of nodules per plant and plant density confirms earlier work such as Abuswar and Mohamed.
Among the growth characteristics, length, fresh weight of shoot, dry weight to froot, number of leaves and roots were higher at 25 J cm)2 whereas the fresh weight of root and dry weight of shoot was higher at 20 J cm)2 laserirradiation.Thelaserirradiation induced significant improvement in seed germination and subsequent growth of seedlings Chen et al.17
Effect of treatments on roots dry weight per twenty plants
For using fresh water, the results were indicated that the root dry weight (RDW) of clover plants were increased of (0.23, 0.24, 0.26, 0.27 and 0.37 g) , (0.36, 0.39, 0.40, 0.52 and 0.66 g) , (0.48, 0.69, 0.76, 0.87 and 1.00 g) , and (0.77, 0.96, 1.11, 1.26 and 1.45 g) for ( N2, Rhizobia, N2+Laser, N2+Rhizobia, and Rhizobia+Laser) for first, second, third and fourth cutting of clover plants, respectively. (Fig. 11). While, there were increased of of (0.16, 0.20, 0.22, 0.23 and 0.31 g), (0.31, 0.33, 0.36, 0.41 and 0.51 g) , (0.39, 0.41, 0.65, 0.81 and 0.96 g) , and (0.59, 0.67, 0.79, 0.99 and 1.23 g) , respectively for root dry weight (RDW) at irrigation by ground water (Figures 11&12).
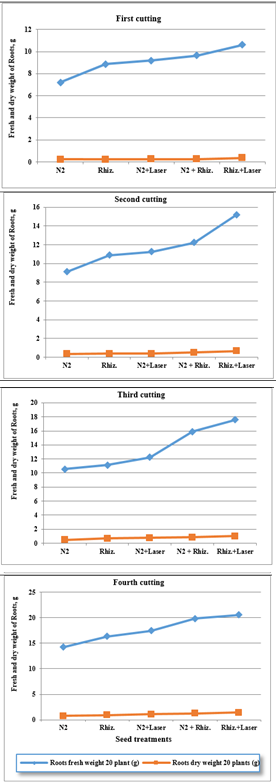
Figure 11 Effect seed treatments on fresh and dry weight roots of plant in clover cultivation at irrigation with fresh water.
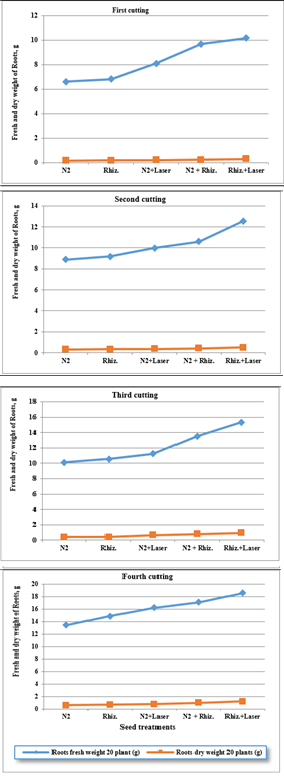
Figure 12 Effect seed treatments on fresh and dry weight roots of plant in clover cultivation at irrigation with ground water.
Positive correlation have been found between root biomass and dry mass yield in alfalfa.59 According to Kusvuran et al.,60 the amount of nitrogen accumulated in alfalfa roots were observed to be much higher than that for other legumes.
Carbon and nitrogen content are good indicators for soil quality. Plants can translocate 20-50% of total fixed carbon to their roots.61 With the maximizing formation of root biomass C inputs into the soil increased. Root biomass contains approximately 40% С and about 18% of the root С for the year could be converted into humus.62
Laser irradiation and Rhizobium inoculation of seeds significantly increased plant height compared to the uninoculated plants (Nitrogen fertilization and or Laser). Plant height was significantly enhanced under Rhizobium inoculation in comparison with nitrogen fertilization.
Vessey & Heisinger63 also found that the accumulation of dry matter in pea was increased after the inoculation with a phosphate solubilizing fungus Penicilliumbilaii.64 found that the cooperation of phosphate solubilizing bacteria and Rhizobium was good to increase the yield of crops. Combined inoculations with N2-fixing and P-solubilizing bacteria were more effective than single inoculation.65
Long irradiation times and high wavelength can cause considerable damage to the seed structures. The physical phenomenon can be observed in the case of seeds when they first absorb the radiation energy and then transform it into chemical energy and use it for subsequent growth.24
In the recent times, pre-sowing stimulation of seeds with laser light has become a popular method of improving the sowing material. This method is especially popular in organic farming, as it is safe for the natural environment.1
Several studies66 performed on various crop plants showed that the effects of laser stimulation and ependon many factors:the wavelength, dose, exposuretime, the species and cultivar of plant.67–70
Govil et al.,66 reported the maximum increase in shoot and root length, dry weight of the seedlings from laser irradiated (30 min) green gram seeds and enhanced emergence, early flowering and maturity, plant height due to the pre sowing He–Ne laser treatment than the control plants off ababean.
To study effect the combination of irradiation laser and incubation rhizobium of clover seeds compared in untreated seeds on the growth parameters of clover seeds. Obtained results were oncluded s following: using treate seeds by combination laser exposed times and Rhizobium gave higher the growth parameters than seeds which treat by the laser or rhizobium or nitrogen only, compared with untreated samples (Control).
The authors confirm that this article content has no conflict of interest, the authors declare that there are no conflicts of interests regarding the publication of this manuscript.
None.

©2023 El-Raouf, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.