Journal of
eISSN: 2373-4310


Research Article Volume 12 Issue 3
Indian Institute of Technology, Kharagpur, India
Correspondence: T.K. Goswami, Indian Institute of Technology, Kharagpur, India
Received: June 25, 2022 | Published: July 11, 2022
Citation: Salim R, Goswami TK. Determination of medicinal properties of black pepper: effect of mortar and pestle crushing, normal grinding and cryogenic grinding. J Nutr Health Food Eng. 2022;12(3):79-85. DOI: 10.15406/jnhfe.2022.12.00360
Cryogenic grinding is now arising as a method to prevent the spice powders from losing its characteristic flavour during storage. The present study is done to determine the medicinal properties of cryogenic ground, ambient ground and crushed samples of black pepper. The solvents used for extraction are hot water, cold water and ethanol. Medicinal properties like antioxidant, anti-diabetic, antimicrobial and anti-cancerous studies are to be determined. All the above studies revealed that cryogenic ground samples are far away much better than ambient ground and crushed samples of black pepper.
Keywords: black pepper, Cryogenic grinding, Anti-oxidant, anti- diabetic, anti-cancerous, anti- microbial
Black pepper (Piper nigrum) is one of the major spices which is used worldwide for both culinary and medicinal purpose. It accounts for about 35% of the world trade. This is the very reason for its name as “king of spices”. Black pepper is an important commodity, since it is valued highly for its flavour, aroma, nutritional and medicinal uses. It is spicy, carminative and aromatic by nature. It contains many essential vitamins and traces of minerals, and amino acids.1
Black pepper has been in existence for more than 4,000 years. Historically, black pepper is used in traditional medicines and home remedies in India. In Ayurveda, Siddha and Unani, black pepper has figured diseases. Piperine is the principle bioactive compound of P. nigrum, which has been reported to have immunomodulatory, anticarcinogenic, antiasthmatic, stimulatory, hepatoprotective, antiulcer activities,2,3 antimicrobial,4 anti-inflammatory.5 Piperine has played significant roles in bioavailability enhancing action of drugs and nutrients.2,3 P. nigrum plays a vast role in improving digestion and it also possesses anti- oxidant activity, anti- diarrhoeal activity, anti- mutagenic activity, anti- depressant activity, anti-platelet activity, anti-hypertensive activity, anti-thyroid activity and anti-asthmatic activity.6 Acetone, ethyl acetate and aqueous extracts of black pepper leaves were evaluated for its anti- microbial studies against clinically important yeast, bacterial and fungal strains using agar disc diffusion method. MIC’s were determined, which showed that acetone and ethyl acetate were more superior in its activity towards the microbes than the aqueous extract.7
From the review of literature collected and studied so far, it was very clear that almost all the medicinal properties of normal ground samples has been determined. But the medicinal properties of cryogenic ground samples were not determined till date. This is a major gap of research and here lies the scope of this project. Therefore, an attempt is made to determine and study all the possible medicinal properties of cryogenic ground black pepper powder and also to compare this medicinal properties with normal ground and crushed samples of black pepper. Thus, the objective of the current study is to determine the medicinal properties (antioxidant, antidiabetic, antimicrobial and anticancerous) of normal ground, cryogenically ground and mortar and pestle crushed samples of black pepper.
Materials
Spice (Black Pepper): Whole black pepper was purchased from the local market of Kharagpur for carrying out the entire study of determining the medicinal properties of black pepper
Sample preparation
About 50 grams of black pepper were conditioned and used for grinding to determine the medicinal properties of black pepper. Black pepper sample was ground using a rotor mill, Fritsch pulverisette, type 14- 702, with sieve size of 0.5 µm. Black pepper was divided into normal or ambient ground, cryogenic ground and mortar and pestle crushed sample.
Preparation of hot extract
About 1g of sample was dissolved in 5ml water and kept in thermomixer at 70ºC for 30 minutes at 750rpm. It was then centrifuged for 10 minutes at 10,000 rpm. The supernatant was then transferred to eppendorf tube and refrigerated. It was then concentrated using a concentrator for 3 hours at 45ºC in aqueous solvent.
Preparation of cold extract
About 1g of sample was dissolved in 5ml water and was kept in dark for 24 hours at room temperature. It was then transferred to eppendorf tube and ultrasonicated for 10 min, centrifuged at 10,000 rpm for 10 min. It was then concentrated using a concentrator for 3 hours at 45ºC in aqueous medium.
Preparation of alcoholic extract
About 1g of sample was dissolved in 5ml ethanol and was kept in dark for 24 hours at room temperature. It was then transferred to eppendorf tube and ultrasonicated for 10mins, centrifuged at 10,000 rpm for 10 minutes. It was then concentrated using a concentrator for 3 hours at 45ºC in alcohol.
Antioxidant studies
Total Phenolic Content (TPC): The total phenol content was determined using Folin- Ciocalteu reagent (FCR) according to the procedure reported by.8 For measuring total phenolic content, gallic acid standard solution in different concentrations were prepared and standard curve of total phenolic content in terms of gallic acid equivalent (GAE) was prepared. A 10 µl sample of hot extract, cold extract and alcoholic extract was taken and 90 µl of distilled water was added to hot and cold extract and 90 µl of ethanol was added to alcoholic extract, making it to a total of 100 µl sample. 200 µl of FCR (10% V/V) was added into each of the extracts. It is then vortexed. 800 µl of Na2CO3 (700mM) was added and incubated in dark at room temperature for 2 hours. After 2 hours, absorbance was measured against reagent blank (alcohol) at 765nm using UV-VIS spectrophotometer. The results were expressed as micrograms of gallic acid equivalent (g/GAE)/ g dry weight of black pepper sample.
FRAP Assay: The working FRAP solution was prepared by mixing 2.5ml FeCl3. 6 H2O, 2.5ml TPTZ, 25ml of acetate buffer, which gives an intense blue colour. Take 997µl working solution and add 3ml of extract. Incubate for 6 minutes at OD 593nm. 997µl of FRAP is added to 3µl solvent as blank. . For construction of the calibration curve, five concentrations of FeSO4, 7H2O were used and the absorbance values were measured as for sample solutions.
DPPH Assay: DPPH solution was prepared in required amount and wrapped with aluminium foil. The initial OD of DPPH solution so prepared was taken against an ethanol blank. The reaction mixture was set by adding 900µl of DPPH and 100µl of sample. The sample was made up to 100µl by taking 10, 20, 30 and 40µl of extract and 90, 80, 70, 60µl of solvent respectively. The solvents used were ethanol for alcoholic extract and distilled water for hot and cold water extracts. OD values were measured at 517nm against a blank of DPPH solution after half an hour of incubation. Percentage inhibition was calculated and IC50 values of the samples were determined.
Percentage inhibition= ((OD of control- OD of sample)/ OD of control) x 100
Antitumour screening studies
Preparation of extract: The concentrated powder of hot water, cold water and ethanol extracts of black pepper were dissolved in Phosphate buffer saline (PBS) and then filtered through 0.2µm syringe filter in a laminar air flow for sterilization and then covered with paraffin wax to avoid any contamination with microbes from the surrounding air.
Anti-bacterial studies
Preparation of various extracts: The aqueous extract of black pepper was prepared by mixing 2g of black pepper powder with 10ml distilled water which was sterilized in a conical flask with occasional shaking. The extract was then filtered using a muslin cloth for large and coarse residue and later filtered using Whatmann No.1 filter paper and stored at 4ºC until used in an air tight container. Ethanol extracts were prepared by mixing 2g of black pepper powder in 10ml ethanol (99%), which was then kept in a rotary shaker for 24h. it was then filtered through Whatmann No.1 filter paper and centrifuged at 10,000rpm for 20 min. The supernatant was then collected and stored in 4ºC until used.
Bacterial cultures: Bacterial strains were selected for determining the antimicrobial study of black pepper samples against pathogenic bacteria, which normally and frequently causes spoilage and deterioration of food. The bacterial strains were purchased from National Collection of Industrial Microorganisms (NCIM), Pune. Gram positive bacteria like Staphylococcus aureus and gram negative bacteria like Pseudomonas aeruginosa and Escherichia coli were used for the study.
Disc diffusion method: Antibacterial activities of the different extracts of black pepper were determined by disc diffusion method as described by Kuhad et al., 2011. The antimicrobial studies were determined using disc diffusion method, in which the black pepper extract diffused through the filter paper discs and reacted with the bacteria which was allowed to grow in nutrient agar.
Bacterial strains were collected from the fresh vial using a sterile loop and it was streaked in nutrient agar (NA) and left for incubation at 30ºC for 24 h. The bacteria got multiplied during this time and it was then transferred into Luria- Bertani broth with 1% agar. Various black pepper extracts at different concentrations of 0.25, 0.5, 1, 2.5, 5, 10, 50, 100 and 200µg/ml were transferred into sterile filter paper disc. This discs were then loaded on the LB agar media with bacterial strains in it. The entire experiment was done in a laminar air flow in a sterile environment. The plates were then incubated for 48 h at 30ºC. The effects were compared with that of ampicillin (0.5mg/ml) as positive control and ethanol and distilled water as negative control. The diameter of zone of inhibition of the study was recorded.
Antidiabetic studies
The anti- diabetic inhibitory activities of ambient ground, crushed ground and cryogenic ground extracts of hot water, cold water and ethanolic extracts of black pepper are evaluated using two anti- diabetic assays at varying concentrations. The two assays used for the study are in- vitro α- amylase inhibitory assay and in vitro α- D glucosidase inhibitory assay. In order to regulate the enzymes like glucosidase and amylase, black pepper extracts are found to produce a large number of protein inhibitors. This study intends to show the minimization of toxicity and side effects of the carbohydrates due to the anti- diabetic activity of black pepper.
Invitro α- amylase inhibitory assay: The reagents for the assay include porcine pancreatic α- amylase of 0.5mg/ml in sodium phosphate buffer, 1% starch in 0.02M sodium phosphate buffer of pH 6.9 with 0.006M sodium chloride and di- nitro-salicylic acid colour reagent. This reagent is prepared by dissolving 1g of 3, 5- di- nitro salicylic acid in 50ml of reagent grade water. Then 30g of sodium potassium tartarate tetrahydrate is slowly added. Then 20ml of 2 N sodium hydroxide is added. Then it is diluted to a final volume of 100 ml with reagent grade water. It is then protected from carbon- di- oxide and stored for no longer than 2 weeks.
The alpha amylase inhibitory activities of the extracts were determined by the modified 3, 5-dinitrosalicyclic acid (DNSA) method of Jain et al.. The assay mixture containing 10 µl of enzyme in 50 µl of 0.02M sodium phosphate buffer and appropriate dilutions of 10 µl of plant extract in different concentration ranges were incubated for 10 min at 25ºC, which was then followed by addition of 500 µl of 1% starch solution. This was incubated for 10min at 25ºC. To this, 1ml DNSA colour reagent was added. It was then incubated using boiling water bath for 5min and then cooled to room temperature, which was then diluted with 10 ml of distilled water. The optical density of all the samples were then measured using UV- VIS spectrophotometer at 540nm. When enzyme was replaced by buffer solution, it was considered as control sample. Percentage inhibition was then calculated. Acarbose (2mg/ml) was used as the positive control, while respective solvents were used as control to ensure that there was no inhibition of alpha amylase. Percentage inhibition was calculated using equation 3.1. The IC50 values were determined from plots of percent inhibition versus inhibitor concentration from the mean inhibitory values. The reference used for alpha amylase inhibitory assay was acarbose purchased from Sigma. The experiments were repeated in triplicate.
Invitro α- D glucosidase inhibitory assay: α- D- glucosidase assay is performed using p- Nitrophenyl- α- D- glucopyranoside as substrate and α- D- glucosidase as the enzyme. Black pepper extract inhibits the activity of the enzyme by forming a yellow coloured complex called p - Nitrophenyl, which is then measured using a spectrophotometer at 405nm. α- glucosidase inhibitory activities of the extracts were determined by the modified method of Pistia- Brueggmann and Hollingsworth (2001). The enzyme extract was prepared by dissolving 7.5µl of enzyme with 400 µl of 0.5 M potassium phosphate buffer at a pH of 6.9. The enzyme extract is then treated with inhibitor at different concentrations of 10-12µg/ml and kept for 10min at 37ºC.100µl of p- Nitrophenyl- α- D- glucopyranoside substrate of 0.03 M is then added and again kept for 10min at 370C. The optical density of the extract is then measured at 405nm using UV-VIS Spectrophotometer. Control samples were prepared without any extract in it. Percentage inhibition was calculated using the following formula. Acarbose (2mg/ml) was used as the positive control, while respective solvents were used as control to ensure that there was no inhibition of alpha amylase. Percentage inhibition was calculated using equation 3.1. The IC50 values were determined from plots of percent inhibition versus inhibitor concentration from the mean inhibitory values. The reference used for alpha glucosidase inhibitory assay was acarbose purchased from Sigma. The experiments were repeated in triplicate.
Antioxidant studies
The antioxidant activity of ambient, cryogenically ground and crushed samples of alcoholic, hot water and cold water extracts were determined using various assays. Results are attached below.
Total Phenolic Content (TPC): The total phenolic content was calculated from a standard gallic acid equivalents curve as shown in Figure 1.
The phenolic content in black pepper extracts reacts with Folin- Ciocalteau reagent to form blue coloured complexes, which are then measured spectrophotometrically at 765 nm. The total phenolic content of hot water, cold water and ethanolic extract of ambient ground, cryogenic ground and mortar and pestle crushed samples were determined. Total phenolic content helps in determining the total phenolic acid concentration present in the sample. Concentration of phenolics in an extract will exhibit directly its antioxidant characteristics. More the total phenolic content, more will be its antioxidant activity and vice-versa. In this study, ethanolic extract of cryogenic ground sample has revealed the highest concentration of phenolic of 560.75 µg/ g of Gallic acid equivalent (GAE). Similarly, normal ground ethanolic and crushed ethanolic extract has shown highest phenolic content of 479.318 and 73.137 µg/g GAE, respectively, which is shown graphically in Figure 2.
DPPH Assay: The standard DPPH free radical scavenging activity of hot water, cold water and ethanolic extract of samples were determined here. IC50 value of each of this sample was determined. IC50 value is the concentration of extract required to reduce the DPPH free radical by 50% of its original concentration. Less the IC50 value, more will be the samples activity to scavenge the DPPH free radical. IC50 values of 2.678, 1.81 and 1.28 mg/ml for cold water, hot water and ethanolic extract of cryogenically ground samples respectively and 2.72, 2.17 and 1.71 for normal ground cold water, hot water and ethanolic extract respectively and 8.78, 8.303 and 6.191 for crushed samples. From cryogenic ground, ambient ground and crushed samples, ethanolic extract is showing the lowest IC50 value, showing that it is having the highest ability to reduce the DPPH free radical.
FRAP Assay: FRAP was calculated from a standard ferrous sulphate curve as shown in Figure 2. FRAP assay deals with the ferric reducing antioxidant power expressed in mM equivalent concentration of FeSO4. FRAP for alcoholic, hot water and cold water extract for ambient ground, cryogenically ground and crushed samples had been performed. The results of these samples are shown in Table 4.3. More the mM concentration, more the extracts antioxidant activity to reduce ferric compounds. From the results, it is clear that ambient ground, cryogenically ground and crushed samples of alcoholic extract is showing the higher values of ferric reducing antioxidant power.
From the results, it is shown that TPC, DPPH and FRAP of ethanolic extracts of ambient ground, cryogenically ground and crushed samples of black pepper are higher than those of hot water extracts followed by cold water extracts. Cryogenic ground samples were showing the highest antioxidant activity than ambient ground sample followed by crushed samples. The reason for this may be because of the greater solubility of phenols present in black pepper in alcoholic extract, leading to its greater antioxidant activity. Liquid nitrogen used for cryogenic grinding would prevent the vapourisation of essential oil during the grinding operation due to which the phenolic acids stayed within the cells of black pepper powder, which in turn increased the antioxidant activity. Since crushed samples were not ground properly and do not give their phenolic acid to react with the assays completely, it gives less antioxidant activity. Thus, it is very clear that from all the anti- oxidant studies, cryogenically ground black pepper extracted with alcohol has exhibited its greater potential as an anti-oxidant agent, which can firth against all the free radicals that are produced in our body as a part of normal metabolic functions.
Antimicrobial studies
The antimicrobial studies of alcoholic extract, hot water and cold water extracts of cryogenic ground, ambient ground and crushed samples were determined. Studies were done using disc diffusion method to determine the zone of inhibition and minimum inhibitory concentration. Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli were used for the determination of antimicrobial activity. Ampicillin was used as positive control at 1.5µg/ml. Alcohol was used as negative control for alcoholic extracts and distilled water was used as negative control for water extract.
Escherichia coli was treated with alcoholic, cold water and hot water extracts of normal ground, cryogenic ground and crushed samples of black pepper extracts. Alcoholic extract of cryogenic ground sample showed minimum inhibitory concentration at 0.25 µg/ml, followed by hot water extract of cryogenic ground and ambient ground sample at 1 µg/ml, followed by crushed sample of cryogenic ground and ambient ground sample of black pepper. Ampicillin and alcohol showed less zone of inhibition. Diameter of zone of inhibition was greater for cryogenic ground alcoholic extract, followed by cryogenic ground hot water, followed by alcoholic extract of ambient ground sample. Among the three strains used for the study, Escherichia coli was destroyed more easily by the black pepper extract with the smallest dose and largest diameter of zone of inhibition (Figure 3–10).

Figure 5 Minimum Inhibitory Concentration of different samples of black pepper extract with Escherichia coli.
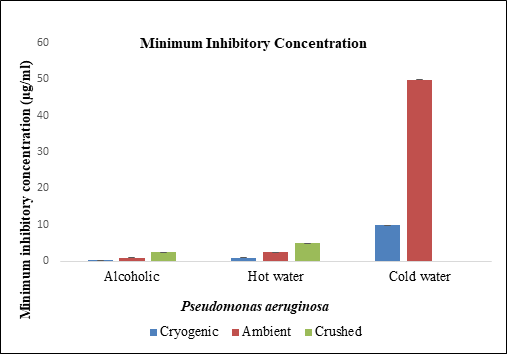
Figure 6 Minimum Inhibitory Concentration of different samples of black pepper extract with Pseudomonas aeruginosa.

Figure 7 Minimum Inhibitory Concentration of different samples of black pepper extract with Staphylococcus aureus.
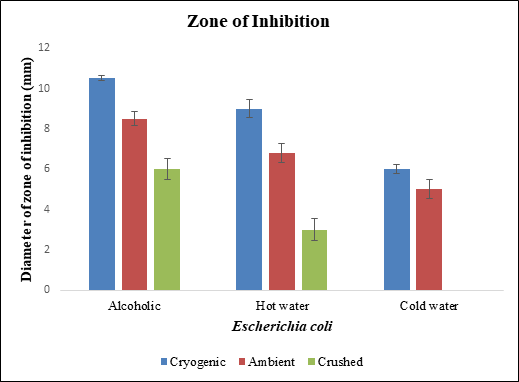
Figure 8 Diameter of zone of inhibition of different samples of black pepper extract with Escherichia coli.
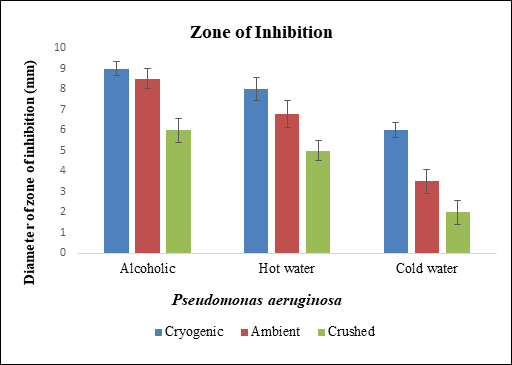
Figure 9 Diameter of zone of inhibition of different samples of black pepper extract with Pseudomonas aeruginosa.
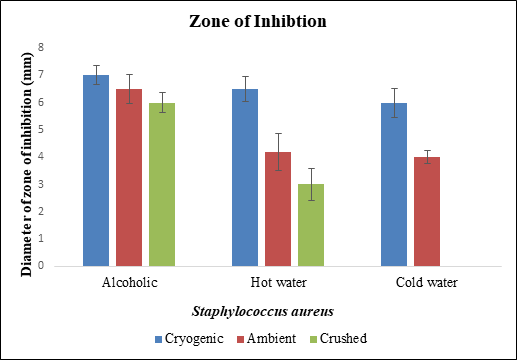
Figure 10 Diameter of zone of inhibition of different samples of black pepper extract with Staphylococcus aureus.
Pseudomonas aeruginosa was treated with hot water, cold water and alcoholic extract of cryogenic ground, ambient ground and mortar and pestle crushed samples of black pepper. Alcoholic extracts showed higher minimum inhibitory concentration at 1 µg/ml followed by ambient ground alcoholic extract. Ampicillin and alcohol showed greater zone of inhibition. Diameter of zone of inhibition was greater for cryogenic ground alcoholic extract, followed by cryogenic ground hot water, followed by alcoholic extract of ambient ground sample.
Staphylococcus aureus was treated with hot water, cold water and alcoholic extracts of cryogenic ground, ambient ground and mortar and pestle crushed samples. Alcoholic extracts of cryogenic ground sample showed minimum inhibitory concentration at 5µg/ml followed by hot water extract of cryogenic ground and alcoholic extract of ambient ground sample at 10µg/ml. Zone of inhibition for alcohol and ampicillin was less. Diameter of zone of inhibition was also greater in alcoholic samples of cryogenic ground, ambient ground and crushed extracts of black pepper.
Anticancer studies
Anti- cancer studies of alcoholic, hot water and cold water extracts of cryogenic ground, ambient ground and crushed samples of black pepper were used. MTT assay carried out for alcoholic, hot water and cold water extracts of cryogenic ground sample, revealed lesser viability in alcoholic extract at 800 µg/ml, followed by alcoholic sample at 400µg/ml followed by hot water extract at 800µg/ml. For normal ground sample, MTT assay showed lesser viability in alcoholic sample at 800µg/ml, followed by alcoholic extract at 400µg/ml, followed by hot water at 400µg/ml. For crushed samples, MTT assay showed lesser viability in alcoholic at 800µg/ml, followed by hot water extract at 800µg/ml, followed by hot water at 400µg/ml. Of the cryogenic ground, ambient and crushed samples, cryogenic ground extract showed greater viability to inhibit the cancerous growth in leukaemic cells. The percentage viability of the extracts are respectively. Graphical representations are shown in Figures 11–13 respectively.
Anti- diabetic studies: The anti- diabetic studies of different extracts of cryogenic ground, ambient ground and mortar and pestle crushed samples of black pepper were determined using α- amylase inhibitory assay and α- D- glucosidase inhibitory assay. All the nine extracts were treated with the two assays for determiming the α- amylase inhibition activity and α- glucosidase inhibition activity
α- amylase inhibitory assay: The anti- diabetic activity of black pepper extractrs were determined using α- amylase inhibitory assay. The enzyme amylase reacts with starch which acts as substrate which is then treated with the colouring reagent DNSA, to form yellow coloured complexes. This colour is then measured with a UV- VIS spectrophotometer at 540nm. Percentage inhibition of all the nine extracts were plotted as a function of concentration. The results showed that out of all the nine extracts of black pepper, cryogenic ground ethanolic extract of black pepper has the highest α- amylase inhibitory activity, with an IC50 value of 0.0253±0.0056µg/ml. Among the hot water extracts, cryogenic ground sample of black pepper showed highest anti- diabetic activity in alpha amylase assay. This extract gave an IC50 value of 0.0397±0.0045µg/ml. Cryogenic ground sample of cold water extract of black pepper also gave the lowest IC50 value of 0.03125±0.0006µg/ml. From this study, it was made clear that cryogenic ground samples of hot water, cold water and ethanolic extract of black pepper gave the highest anti- diabetic activity of black pepper. IC50 values of the samples are given. Graphical representations are shown in Figure 14.
α- glucosidase inhibitory assay: α - D- glucosidase assay is performed using p- Nitrophenyl- α- D- glucopyranoside as substrate and α- D- glucosidase as the enzyme. Black pepper extract inhibits the activity of the enzyme by forming a yellow coloured complex called p - Nitrophenyl, which is then measured using a spectrophotometer at 405nm.The anti- diabetic activity of black pepper extractrs were determined using α- D- glucosidase inhibitory assay. Percentage inhibition of all the nine extracts were plotted as a function of concentration. The results showed that out of all the nine extracts of black pepper, cryogenic ground ethanolic extract of black pepper has the highest α- glucosidase inhibitory activity, with an IC50 value of 0.033±0.006µg/ml. Among the hot water extracts, cryogenic ground sample of black pepper showed highest anti- diabetic activity in α- D- glucosidase assay. This extract gave an IC50 value of 0.05±0.014µg/ml. Cryogenic ground sample of cold water extract of black pepper also gave the lowest IC50 value of 0.067±0.002µg/ml. From this study, it was made clear that cryogenic ground samples of hot water, cold water and ethanolic extract of black pepper gave the highest anti- diabetic activity of black pepper. IC50 values of the samples are given. Graphical representations are shown in Figure 15.
From this study it was clear that, the cryogenic ground sample has exhibited greater potential of anti-diabetic activity in both α- amylase assay and α- glucosidase assay. Thus, cryogenic ground extract can be best considered as a natural agent against amylase and glucosidase,that are responsible for increasing the blood sugar levels, due to its higher anti-diabetic activity (Figure 2–15).9–36
From all the studies, it was very clear that cryogenic ground sample of black pepper extracted with alcohol exhibited greater medicinal properties than any other sample of black pepper. The reason for the greater medicinal property of this extract may be because of the greater solubility of phenols present in black pepper in alcoholic extract. Liquid nitrogen used for cryogenic grinding would prevent the vaporisation of essential oil during the grinding operation due to which the phenolic acids stayed within the cells of black pepper powder. Since crushed samples were not ground properly and do not give their phenolic acid to react with the assays completely, it gives less antioxidant activity. Since phenolic extract are more soluble in alcohol than in water extract, higher medicinal properties are obtained in alcoholic extract. When cryogenic ground sample and alcoholic solvent comes together, they contribute to a larger extent to form a superior extract.
None.
The author states there are no conflicts of interest.
None.

©2022 Salim, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.