Journal of
eISSN: 2373-4310


Research Article Volume 8 Issue 1
Department of Food Science and Technology, Federal University of Technology, Nigeria
Correspondence: Ojo DO, School of Agriculture and Agricultural Technology, Department of Food Science and Technology, Federal University of Technology, Akure, Ondo State, Nigeria, Tel +234 803 478 0116
Received: October 13, 2017 | Published: February 9, 2018
Citation: Ojo DO, Enujiugha VN. Comparative evaluation of ungerminated and germinated co-fermented instant ‘OGI’ from blends of maize (Zea mays) and ground bean (Kerstingiella Geocarpa). J Nutr Health Food Eng. 2018;8(1):68-73. DOI: 10.15406/jnhfe.2018.08.00258
‘Ogi’ is a fermented maize food commonly consumed as a weaning and breakfast diet in sub-Saharan Africa. Instant ‘ogi’ production arises from the growing demand for fast foods among its urban populace. The study was carried out to determine the effect of different treatments on the proximate, mineral, anti-nutrient, microbial and sensory quality of 30% substituted Kerstingiella geocarpa ‘ogi’. Four samples of 30% Kerstingiella geocarpa ‘ogi’ were subjected to different treatments and coded USF, GSF, GPF, UPF. Sample USF (control) was ungerminated and spontaneously co-fermented while sample GSF was germinated and spontaneously co-fermented; Sample GPF was germinated and probiotic co-fermented while sample UPF was ungerminated and probiotic fermented. Results of analysis showed that the germinated samples (GPF, GSF) had higher protein, ash and crude fibre contents (P>0.05) but lesser fat content than their ungerminated co-fermented counterpart. Except for manganese content, the mineral contents (Ca, P, K, Mg, Fe, Zn, Cu) of the germinated spontaneously co-fermented sample, GSF were higher than that of the germinated probiotic co-fermented sample, GPF. Trypsin inhibitor content ranged from 1.20-1.54mg/100g and was significantly lower in the germinated samples than their ungerminated counterpart. Results of sensory evaluation shows that sample USF (control) had the highest score of 8.78 for aroma while sample GPF scored highest for taste and overall acceptability. Therefore, germination and probiotic co-fermentation could be adopted as an improved treatment for commercial production of 30% Kerstingiella geocarpa-maize ‘ogi’.
Keywords: fermented maize, Kerstingiella geocarpa, probiotic fermentation, spontaneous fermentation
USF, ungerminated and spontaneous fermented; GSF, germinated and spontaneously fermented; GPF, germinated and probiotic fermented; UPF, ungerminated and probiotic fermented; MRS, man rogosa sharpe
Fermented maize product ‘ogi’ is a popular weaning and breakfast cereal in sub-Saharan Africa traditionally made from maize, sorghum or millet. It is prepared by steeping clean grains in water at room temperature (25±2 0C) for two to three days.1 The steep water is decanted and the fermented grain is washed with clean water and then wet-milled. The bran is removed by wet sieving and the slurry is allowed to settle and ferment spontaneously for another two to three days.
Maize grains are naturally deficient in lysine and tryptophan, amino acids essential for growth of infants and pre-school children. Efforts to improve the nutritional status of these staples have therefore been based on fortification with legumes to provide the deficient amino acids.2 Attempts that have been made for nutrient restoration and fortification of ‘ogi’ include blending with fermented and unfermented legumes.3,4 Although, previous studies has shown ‘ogi’ with acceptable sensory characteristics without a fermentation stage, the nutritional quality of the ‘ogi’ is low compared to their fermented counterpart.5,6
Protein malnutrition is still a serious problem for a large segment of the population in developing countries conditioned by poor quality and quantity of protein in their diets. Kerstingiella geocarpa Harms, an indigenous tropical crop and a promising alternative source of high quality protein for food and feed for the tropics can be incorporated into the diet.7 It can be utilized in weaning foods, snack and diet for different groups. The Kerstingiella geocarpa (ground bean) is known to be rich in essential minerals and amino acids.8 Its anti - nutritional constituents are usually removed during hydrothermal treatment, soaking and fermentation which the seeds are subjected to during processing.9 The anti - nutrients could also be drastically reduced by germination of the seeds. According to V Ramakrishna10 germination is a more effective method in reducing trypsin inhibitor activity, tannins, polyphenols and phytic acid than various cooking treatments.
Previous work by MO Aremu et al.11 in which ‘ogi’ was substituted with 10%, 20%, 30% and 40% Kerstingiella geocarpa and co-fermented with the beans has shown that 30% substitution of ‘ogi’ flour can be maintained with higher nutrient contents. Therefore, the objectives of the study were to determine the effect of different treatments of germination and co-fermentation on the proximate, mineral, anti-nutrient, and sensory qualities of 30% substituted Kersting’s groundnut ‘ogi’.
Source of Materials
Fresh yellow maize (Zea mays) and ground bean seeds (Kerstingiella georcarpa) were purchased from the ‘Oba’ market, Akure, Ondo state, Nigeria.
Preparation of Samples
Maize grains and ground bean seeds were sorted to remove spoilt grains and other foreign materials.
Isolation of indigenous starter culture- Lactobacillus platarum: Steep water from spontaneously fermenting maize was collected aseptically into a sterile bottle for serial dilution using the pour plate technique. 1ml of the steep water was homogenized with 9ml of sterile diluents (5g peptone, 8.5g NaCl, 1 L distilled water) and serial dilution was carried out using the pour plate technique. Media used was sharpe agar and 10ppm of cycloheximide (Merck, Darmstadt, Germany) was added to the media to prevent growth of yeasts and moulds. The plates were cultured anaerobically in an anaerobic jar. Each of the colonies was further streaked on DeMan Rogosa Sharpe (MRS) agar to obtain pure strains of Lactobacillus plantarum.
Processing of maize grains (Zea mays): Ungerminated maize grains were soaked in distilled water in the ratio of 1:3 (w/v) grains to water at room temperature of 28±2 0C for 8h prior to spontaneous fermentation while the probiotic fermented ones were sterilized and inoculated with Lactobacillus plantarum prior to probiotic fermentation.
Germinated maize grains were spread on jute bags, covered with moist muslin cloth and left to germinate for 72 h and distilled water was sprinkled on the seeds every 12h until the end of germination period. The germinated seeds were then washed, oven dried at 500C for 24h and ruffled to remove the sprouts. The grains were then stored in labeled polyethylene bags prior to spontaneous/probiotic fermentation.6 Probiotic fermented grains were milled, sieved to remove shaft and sterilized at 1710C for 30min and cooled, the slurry was then inoculated aseptically with 1 ml of the innoculum starter organism containing 2.0x109cfu/ml of Lactobacillus plantarum.
Processing of Ground bean (Kerstingiella georcarpa): Ungerminated ground bean seeds were soaked in distilled water in the ratio of 1:3 (w/v) grains to water at room temperature of 28±2 0C for 8h and dehulled prior to spontaneous fermentation while the probiotic fermented ones were sterilized and inoculated with Lactobacillus plantarum prior to probiotic fermentation.
Germinated ground bean seeds were spread on jute bags, covered with moist muslin cloth and left to germinate for 72h and distilled water was sprinkled on the seeds every 12h until the end of germination period. The germinated seeds were then washed, dehulled and oven dried at 500C for 24h. The grains were then stored in labeled polyethylene bags prior to spontaneous/probiotic fermentation.12 Probiotic fermented grains were milled, sieved to remove shaft and sterilized at 1710C for 30min and cooled, the slurry was then inoculated aseptically with 1 ml of the innoculum starter organism containing 2.0x109cfu/ml of Lactobacillus plantarum.
Blend formulation: Four samples coded USF, UPF, GPF and GSF were formulated from maize and ground bean grains in the ratio 70:30w/w and thereafter subjected to different combined treatments of germination and co-fermentation. The control sample, USF was made from blends of ungerminated maize and ungerminated ground bean which were spontaneously co-fermented while Sample UPF was formulated from blends of germinated maize and germinated ground bean which were spontaneously co-fermented. Sample GPF was formulated from blends of germinated maize and germinated ground beans which were subjected to probiotic co-fermentation and sample GSF was formulated from blends of ungerminated maize and ground beans subjected to spontaneous co-fermentation.
Analysis
Proximate chemical compositions of the samples were determined using the methods of13 Carbohydrate content was determined by substracting the sum of the percentage weight of crude protein, crude fibre, ash, fat from 100 percent.
The minerals were analysed by dry-ashing the samples at 5000C and dissolving the ash in volumetric flask using deionised water with a few drops of concentrated hydrochloric acid. The minerals calcium, iron, phosphorus, zinc, copper, magnesium and manganese were determined using atomic absorption spectrophotometer (Perkin - Elmer Model 403, Norwalk, CT, USA). Sodium and potassium were determined using a flame photometer (Model 405, Corning, UK). For anti - nutrient determination, phytic acid contents were determined by the method described by EL Wheeler14 tannin contents were determined by the method of15 and oxalate contents were determined by the method of (Figures 1‒4).16
Sensory evaluation was carried out using a 9 point Hedonic scale as reported by MO Iwe.17 The maize - ground bean ‘ogi’ samples were made into a thin gruel and evaluated for taste, appearance, aroma, and overall acceptability by a panel of ten members using a 9-point Hedonic scale. The hedonic ratings on a scale of 1-9 were: 1=dislike extremely, 5=neither like nor dislike 9=like extremely. The data obtained were analyzed using a one-way Analysis of Variance and the means separated by Duncan New Multiple Range Tests (DMNRT) at 5% significance level (SPSS version 19 computer software).18

Figure 1 Processing method adapted for the production of ungerminated, spontaneous fermented maize -ground bean ‘ogi’ (Control).
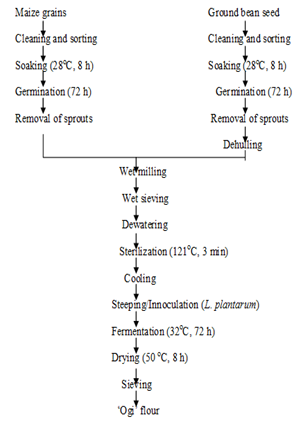
Figure 2 Processing method adapted for the production of germinated, spontaneous fermented maize -ground bean ‘ogi’ (GSF).
Table 1 show the proximate composition of ‘ogi’ from blends of maize and ground bean. A higher ash content was observed in the germinated ‘ogi’ samples (GSF and GPF) than in the ungerminated samples (USF and UPF). The increase may be due to the activities of the endogeneous enzymes which hydrolyses the complex organic compounds to release more nutrients.19
|
Sample |
USF |
GSF |
GPF |
UPF |
|
Moisture |
8.30±0.02d |
8.50±0.02c |
9.00±0.04a |
8.60±0.02b |
|
Ash |
1.30±0.01c |
2.70±0.01a |
2.23±0.00b |
1.10±0.00d |
|
Crude Fibre |
2.33±0.03d |
2.62±0.01b |
2.83±0.01a |
2.39±0.02c |
|
Fat |
5.44±0.01a |
4.30±0.01c |
4.10±0.01d |
5.19±0.01b |
|
Protein |
9.86±0.04d |
11.00±0.02b |
11.30±0.12a |
10.76±0.10c |
|
Carbohydrate |
72.77±0.12a |
70.88±0.12c |
70.54±0.20d |
71.96±0.11b |
Table 1 Proximate Composition of ‘Ogi’ formulated from blends of maize and ground bean (%)
Values with different superscript in a row are significantly different (p>0.05)
Values are means±standard deviation from duplicate determinations
Key: USF, ungerminated and spontaneous fermented sample (control); GSF, germinated and spontaneously fermented sample; GPF, germinated and probiotic fermented sample; UPF, ungerminated and probiotic fermented sample.
The fibre content of the germinated samples (GSP and GPF) was higher than the ungerminated samples (USF and UPF). This could be due to the endogenous enzymes hydrolyzing the complex carbohydrate to release fibre.20 The control sample (USF) had the least crude fibre content and the highest carbohydrate content while the sample GPF had the highest crude fibre and least carbohydrate contents. This shows that an inverse relationship exists between crude fibre and carbohydrate contents of the formulated samples.
The inclusion of ground bean in the formulation increased the fat content of the samples. The fat content of the ‘ogi’ samples were lesser in the germinated ‘ogi’ samples (UPF and GSF) than the ungerminated samples;21 reported a reduced fat content in malted millet for ‘ogi’ production. The observed decrease in fat content of germinated samples might be due to the increased activities of the lipolytic enzymes during germination.22 The observed lower lipid levels in the germinated samples could improve the shelf stability of the ‘ogi’ during storage.
The formulated blends had high protein contents. This is most probably due to the inclusion of ground bean in the formulation. The germinated ‘ogi’ samples had higher levels of protein reaching up to 11.30% in the germinated probiotic co-fermented sample (GPF) and 11.00% in the germinated spontaneous co-fermented sample (GSF). The least value of 9.86% was obtained in the ungerminated spontaneous co-fermented sample. The increase in protein could be attributed to a net synthesis of enzymic protein by the seeds during germination23,24 also reported that the increase in protein on germination of corn seed was due to mobilization of storage nitrogen producing the nutritionally high quality proteins which the young plant needs for its development.
From the results in Table 2, there were significant differences in the mineral content of the samples. Calcium content of the samples was between 105 and 119mg/100g; potassium content ranged from 75.12-87.10 mg/100g; iron content ranged from 4.2-5.6mg/100g and Zn content ranged from 1.6-2.7mg/100g. Except for sodium, the mineral content of the germinated samples were observed to be higher than in the ungerminated samples. This could be due to release of more minerals25 and subsequent reduction in sodium content occurring during germination. Based on the Food Drug Administration’s (FDA) recommended dietary allowance, calcium, magnesium, Iron, Zinc requirement of infant are 210mg/day, 30mg/day, 0.27mg/day and 2mg/day respectively. Hence, the formulated samples would significantly contribute to the infant’s calcium, magnesium, iron, and zinc intake if used as a weaning diet. Table 2 shows the mineral composition of ‘ogi’ from blends of maize and ground beans.
|
Minerals |
USF |
GSF |
GPF |
UPF |
|
Macro Minerals |
||||
|
Ca |
113.00±0.01c |
121.52±0.02a |
119.31±0.02b |
105.00±0.01d |
|
P |
84.87±0.01c |
91.96±0.01a |
91.40±0.01b |
81.04±0.36d |
|
K |
75.12±0.12d |
87.10±0.12a |
82.70±0.21b |
81.90±0.23c |
|
Mg |
375.30±0.21c |
445.80±0.11a |
440.70±0.22b |
333.40±0.24d |
|
Na |
6.15±0.00c |
5.12±0.01b |
5.14±0.01b |
6.51±0.01a |
|
Micro Minerals |
||||
|
Fe |
4.6±0.01b |
5.1±0.01a |
5.6±0.00c |
4.2±0.01d |
|
Zn |
1.6±0.01c |
2.7±0.00a |
2.2±0.01b |
1.9±0.01d |
|
Cu |
13.1±0.01c |
16.5±0.01a |
14.1±0.01b |
11.5±0.01d |
|
Mn |
1.67±0.01c |
1.24±0.01a |
1.06±0.01b |
0.77±0.01d |
Table 2 Mineral Composition of ‘Ogi’ formulated from blends of maize and ground bean (mg/100g)
Values with different superscript in a row are significantly different (p>0.05)
Values are means±standard deviation from duplicate determinations
Key: USF, ungerminated and spontaneous fermented sample (control); GSF, germinated and spontaneously fermented sample; GPF, germinated and probiotic fermented sample; UPF, ungerminated and probiotic fermented sample.
The anti - nutrient content of ‘ogi’ formulated from blends of maize and ground bean is shown in Table 3. There were no significant differences in the tannin content of the ‘ogi’ samples. The germinated probiotic fermented sample (GPF) had the least trypsin inhibitor and phytate contents. Oxalate content was low in sample GPF but the least content was observed in sample UPF. The result is similar to the findings of26 who observed that probiotic fermentation of finger millet (Eleusine coracane) with starter culture from previously fermented finger millet achieved a desirable goal of reduced tannin and phytate when compared to uncontrolled fermentation.
|
Sample |
USF |
GSF |
GPF |
UPF |
|
Tannins |
0.14±0.01a |
0.13±0.00b |
0.13±0.01b |
0.14±0.00a |
|
Trypsin inhibitor |
1.54±0.02a |
1.38±0.02b |
1.20±0.01d |
1.30±0.01c |
|
Phytate |
0.78±0.03a |
0.70±0.00b |
0.64±0.01d |
0.66±0.02c |
|
Oxalate |
0.45±0.01a |
0.42±0.01b |
0.36±0.01c |
0.33±0.00d |
Table 3 Anti - Nutrients of ‘Ogi’ formulated from blends of maize and ground bean (mg/100g)
Values with different superscript in a row are significantly different (p>0.05)
Values are means±standard deviation from duplicate determinations
Key: USF, ungerminated and spontaneous fermented sample (control); GSF, germinated and spontaneously fermented sample; GPF, germinated and probiotic fermented sample; UPF, ungerminated and probiotic fermented sample.
A thin gruel of ‘ogi’ was prepared by vigorously mixing the formulated ‘ogi’ in water in the ratio 1:4w/v and then stirring under low heat until gelatinization occurred. Sensory panels of 22 semi-trained panelists were set up to assess some of the attributes of the gruel when served warm. In terms of colour, the ungerminated samples (USF and UPF) had higher scores than the germinated samples (GSF and GPF). In terms of aroma, the ungerminated spontaneous fermented/control sample (USF) was best rated followed by the germinated spontaneous fermented sample (GSF). The high aroma score of sample USF could be due to the presence of Corynebacterium in spontaneous fermentation hydrolysing the ‘ogi’ starch to organic acids and the presence of yeast (Saccaromyces cerevisae) and fungi (Candida mycoderma) which contribute to flavour development.27 The probiotic samples (GPF and UPF) were rated best in terms of taste and overall acceptability. The result of the sensory score of ‘ogi’ gruel formulated from blends of maize and ground bean is seen on Table 4.
|
Attributes |
USF |
GSF |
GPF |
UPF |
|
Colour |
7.89b |
5.22d |
7.33c |
8.86a |
|
Aroma |
8.78a |
7.78b |
7.33c |
6.56d |
|
Taste |
7.33c |
6.67d |
8.67a |
7.89b |
|
Overall acceptability |
7.67c |
6.78d |
8.98a |
8.67b |
Table 4 Sensory evaluation of ‘ogi’ gruel formulated from blends of maize and ground bean
Values with different superscript in a row are significantly different (p>0.05)
Values are means±standard deviation from duplicate determinations
Key: USF, ungerminated and spontaneous fermented sample (control); GSF, germinated and spontaneously fermented sample; GPF, germinated and probiotic fermented sample; UPF, ungerminated and probiotic fermented sample.
It can be concluded that germination and co-fermentation improved the nutritional qualities of 30% Kerstingiella geocarpa substituted ‘ogi’. In terms of consumer acceptance, the germinated samples had higher level of acceptance than the ungerminated ones but the germinated probiotic sample had the highest acceptability score. Therefore, germination and probiotic co-fermentation could be adopted as an improved treatment for commercial production of 30% Kerstingiella geocarpa-maize ‘ogi’.
None.
Author declares that there is no conflict of interest.

©2018 Ojo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.