Journal of
eISSN: 2373-4310


Research Article Volume 1 Issue 1
Department of Food Engineering, Izmir Institute of Technology, USA
Correspondence: Sebnem Tellioglu Harsa, Department of Food Engineering, Izmir Institute of Technology, Gulbahce Mh. Izmir Yt Pk 35430 Urla/Izmir, Turkey, Tel 00902327506291, Fax 00902327506196
Received: April 12, 2014 | Published: April 25, 2014
Citation: Cabuk B, Tari C, Harsa ST. β -Galactosidase immobilization on chitosan-hydroxyapatite complex: effects of immobilization conditions. J Nutr Health Food Eng. 2014;1(1):13-23. DOI: 10.15406/jnhfe.2014.01.00004
In this study, β-galactosidase from Kluyvermoyces lactis was immobilized onto chiosan-hydroxyapatite. Hydroxyapatite (HAP) is a nontoxic ceramic biomaterial generally that has been used for dental applications, drug delivery, immobilization of metals, enzymes and recently some yeast cells. Effects of immobilization parameters including medium temperature and pH, enzyme concentration and coupling time was evaluated to obtain the best reaction conditions. The optimal immobilization pH was determined as pH7.5 with the highest enzyme activity of 1337.2Ug-1 chitosan-HAP beads and highest immobilization efficiency of 81.4%. Furthermore, the immobilized protein amount showed increase with increasing enzyme concentration as expected. However, highest specific activity (365.2mg-1 protein) was achieved at low enzyme concentrations. Characterization of immobilized enzyme was tested using o-nitrophenol-β-D-galactopyranoside (ONPG) as a substrate. Immobilized enzyme exhibited better thermostability and higher stability at lower (pH 5.0) and higher (pH 9.0) pH values. Immobilization also resulted in a shift of 0.5units in pH optimum for maximum enzyme activity. In contrast to optimum pH value, after immobilization no shift occurred in optimum temperature. Furthermore enzyme retained 81.8% of its initial activity during consecutive eight cycles. After 15days of storage, immobilized enzyme obtained 82.6% of its original activity, whereas free enzyme showed a significant decrease.
Keywords: chitosan, hydroxyapatite, immobilization, lactase
Improving enzyme stability has a noticeable importance for the growing enzymatic applications in food industry. For this reason, development of suitable heterogeneous systems is still in great attention as it provides recycling and separation of catalyst from reaction mixture, continuous flow and increased operational stability. Immobilization has been used to combine enzyme catalysts into/onto solid supports and producing heterogeneous system for industrial uses. Chitosan is one of the most widely used natural polysaccharide used for immobilization of enzymes due to its water soluble, porous, inert and biocompatible structure. However, chitosan can easily be deformed physically during use and this can be improved by modification of chitosan by complex formation with solid type powders. For this purpose, complex formation of chitosan with different types of materials has been increasingly studied.
Hydroxyapatite (HAP) is a non toxic ceramic biomaterial generally preferred to form rigid composites due to its bioactive properties. In addition to its nontoxicity, HAP is biodegradable and has been used for dental applications, drug delivery, immobilization of metals, enzymes and recently some yeast cells. The use of chitosan-HAP complexes was mostly examined as an implant material in the field of bone tissue engineering and dental treatments. Venkatesan et al.,1 studied bone grafting characteristics of chitosan-HAP composites in the presence of amylopectin and chondroitin sulfate.
β-galactosidase simply known as lactase is a significant enzyme in food industry mainly used to hydrolyse lactose in milk. In addition to provide lactose free milk for lactose intolerant people, hydrolysis of lactose avoids textural problems in frozen milk products. β-galactaosidase from different sources has been immobilized on many various type of matrixes including chitosan, alginate, sepabeads and membrane type carriers.2‒5
The formation of a suitable heterogeneous matrix depends on many factors such as reaction temperature and pH, catalyst concentration and its mixing ratio, duration of the immobilization. Therefore in the present study, we aimed at understanding the relationship between immobilization parameters and immobilization efficiency.
β-galactosidase from K. lactis (G-3664,3000 U.mL-1), glutaraldehyde (grade II,%25), sodium tripolypahosphate and chitosan (>90 purity) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). And all other chemicals were obtained from Merck AG (Darmstdat, Germany).
Preparation of glutaraldehyde activated chitosan-hydroxyapatite (chitosan-HAP) microspheres
As supports for enzyme immobilization, equal amounts of powdered chitosan and HAP were dissolved in 1M of 100ml acetic acid solution. This solution was added drop wise through a syringe into gently stirred 1.5% w/v sodium tripolyphosphate crosslinking solution. Thereafter, the beads were washed with deionized water until neutrality. For activation, chitosan-HAP beads were incubated in 0.1% glutaraldehyde solution for 24hours under constant stirring. The excess glutaraldehyde was washed off and suspended in 0.025M of phosphate buffer at pH 6.86.
Immobilization
1.5g of support was added to 5ml β-galactosidase enzyme solution in 0.025mM of phosphate buffer at pH 6.86. The preparations were left at 20°C under continuous and gentle stirring (150rpm) for 24hours. The preparations obtained were washed with the buffer until no enzyme remained in the washing solution.
Optimization of the immobilization conditions
Optimum conditions for immobilization were determined by changing the conditions individually. The immobilization was carried out over the temperature range between 10-30°C, the pH range between 4.0 and 9.0, the adsorption time between 0 and 24h and the concentration of β-galactosidase between 0,23-2,57x103mgg-1 to determine the immobilization conditions.
Determination of the immobilized protein amount
The efficiency of enzyme immobilization was estimated on the basis of the difference between the amount of protein amount added to the beads and that recovered in the pooled supernatant and washing fractions. Protein content of the enzyme solutions was determined according to the method of Bradford.6 Bovine Serum Albumin (BSA) was used as a standard.
Determination of activity
The activity of β-galactosidase was carried out according to the procedure described in the Food chemical codex7 using the chromogenic substrate o-nitrophenol-β-D-galactopyranoside (ONPG) (8.3mM) prepared in 0.02M phosphate buffer (pH 6.5) enriched withmgCl2. The amount of substrate and enzymes used were 4ml and 1ml, respectively. At time zero, 1ml of enzyme solution was added to ONPG solution and incubated at 37°C for 15minutes for the reaction. The reaction was stopped by the addition of 1ml of reaction mixture to the tubes containing 1ml of 10% sodium carbonate and the addition of 8ml deionized H2O. The procedure was followed by reading the absorbance at 420nm. One unit of enzyme activity was defined as that quantity of enzyme that would liberate 1.0μM of O-nitrophenol from ONPG per minute under the conditions of assay.
For the activity of immobilized enzyme the assay method was essentially the same as for the native enzyme, except that the immobilized enzyme suspension was used instead of free enzyme and that the reaction mixture was incubated with shaking.
The temperature profiles and thermal stability of free and immobilized β-galactosidase
Influence of temperature on activities of free and immobilized β-galactosidase were investigated by measuring the activities at different temperatures ranging from 20-55°C.
Thermal stability were measured by incubating the free or immobilized enzyme at various temperatures ranging from 20-55°C for 30minutes in 0.02M phosphate buffer at pH 6.5 then measuring the residual activities under standard assay conditions.
The pH profiles and pH stability of free and immobilized β-galactosidase
Effect of pH values on the activities of free and immobilized β-galactosidase were carried out at pH range 4.0-9.0 using the following buffer systems; sodium acetate (4.0, 5.0), potassium phosphate (pH 6.0, 6.5, 7.0, 7.5) and Tris-HCl (pH 8.0, 9.0).
The pH stabilities of free and immobilized enzyme were determined by preincubation of the enzyme at different pH values at 37°C for 30min, followed by activity measurement.
Determination of kinetic parameters
Kinetic parameters, Km and Vmax, for free and immobilized enzyme were carried out at the substrate concentration in the range 0.5-12.5mM. The rate of enzymatic hydrolysis of ONPG can be expressed by Line weaver-Burk double reciprocal plot.
Operational and storage stability of the immobilized enzyme
The operational stability was tested by repeated batch experiments using the method for activity determination. After each reaction run, immobilized beads were removed and washed with buffer to remove any residual substrate within the beads, then reintroduced to fresh reaction medium.
The storage effect on the activity was evaluated by measuring the residual activities in a batch operation mode after storage in phosphate buffer at 4°C for 15days.
Effect of process parameters on immobilization efficiency and activity
Medium temperature: Effect of different medium temperatures on immobilization efficiency, enzyme activity and specific activity is shown in Figure 1. Results demonstrated that increasing temperature of the medium resulted in an increase in immobilization efficiency. Highest amount of enzyme with 82.3% efficiency was obtained at 30°C but the specific activity of the immobilized enzyme was found to be around 50.2 Umg-1 protein. It appeared that temperature above 20°C had a negative impact (p<0.05) on immobilization; causing some conformational changes due to thermal denaturation resulting in a decrease in specific activities. Therefore according to the results, enzyme with the highest specific activity of 195.2 Umg-1 proteins was immobilized at the optimal medium temperature of 20°C. The decrease with increasing medium temperature is consistent with the study conducted by Vasileva et al.8 They used modified polypropylene membrane as a support material for enzyme immobilization. Amount of bound protein and enzyme activity was recorded to decrease above 4°C. Similarly, Jochems et al.9 investigated the effect of temperature on enzyme immobilization and observed an increase in immobilization yield with increasing temperature up to 60°C, but above 25°C, a drastic decrease in enzyme activity was observed.
Effect of medium pH on immobilization and catalytic activity: In the present study, the effect of pH was investigated in the range of 4.0–9.0 using different buffer solutions. Figure 2 indicated the change in activities and immobilization efficiency values of immobilized β-galactosidase enzyme with varying pH values. It clear that pH of the immobilization medium had a significant effect (p<0.05) on enzyme activity and immobilization yield. Generally, it is expected to obtain the highest specific activity of immobilized enzyme at or around the optimum pH level. Results indicated that immobilization efficiency followed the same trend with both activity values, yielding a peak of 81.4% at pH 7.5 with the maximum enzyme activity of 1337.2 Ug-1 chitosan-HAP beads. Moreover, specific activity was also had the highest value (370.0 Umg-1 protein) at pH 7.5 and below 50 Umg-1 protein was observed below pH 5.0. Covalent binding between the activated support and an enzyme is mainly based on the reaction between the primary amino groups, usually lysine residues located on the surface of the enzyme and the aldehyde groups of glutaraldehyde introduced to the support previously. The loss in enzymatic activity at too high and too low pH values might be contributed to the alterations in the enzyme conformation resulting in decrease in immobilization efficiency. Similarly, study of Liu et al.,10 showed that immobilization of β-galactosidase from Klyveromyces fragilis is significantly (p<0.05) depended on the medium pH and between pH 6.0 to 9.0, with the optimum pH 7.0, higher enzyme loadings were achieved. Therefore, in our study, pH 7.5 was established as the optimal pH of the immobilization system.
Effect of coupling time on immobilization and catalytic activity: As can be seen from Figure 3, immobilization efficiency indicated that significant amount of enzyme was bound to Chitosan-HAP beads within firsthour whereas specific activity continues to increase significantly (p<0.05) with time. In contrast to efficiency values, both enzyme activity and specific activity increased significantly showing an increase in amount of enzyme immobilized and reached maximum values within ninehours. Optimum immobilization yield and specific activity were calculated as 91.3% and 432.7 Umg-1 protein respectively at 9th hour. This is not an expected result since as the duration of coupling rises, increase in the extent of multipoint interactions between the support and enzyme resulting in a decrease in the enzymatic activity has been reported earlier in many studies.11‒13 The results may be explained by the case that adsorption of the enzyme into porous support quickly occurred and covalent bonds acted as a barrier for substrate diffusion leading to a lower enzyme activity. Within time as the covalently bound enzyme amount increased, both activity values increased and at 9thhour, linkage reaction was almost completed.
Effect of enzyme concentration on immobilization and catalytic activity: In order to achieve high levels of immobilization, effects of varying enzyme concentration ranged between 0.23-2.57g enzyme g-1 wet beads were investigated. It seems that the increase in the initial enzyme concentration resulted in an increase in the activity of immobilized β-galactosidase (Figure 4). As seen, the amount of bound enzyme increased with the increase in enzyme concentration up to 23.0mg of protein/g of support (2.57g enzyme g−1 support). In contrast, specific activity of the immobilized enzyme and the immobilization efficiency were not correlated with the amount of enzyme bound; while the enzyme concentration increased from 0.23 to 2.57 g g-1 support, the unbound enzyme amount decreased in addition to specific activity (365.2 Umg-1 protein to 168.8 Umg-1 protein) (Figure 5). Thus, it seems that specific activity was negatively affected (p<0.05) by enzyme concentration. Maximum specific activity (365.2 Umg−1 protein) achieved working at low estenzyme concentration (0.23g g-1 support). It was assumed that the diffusion of the substrate got limited as the enzyme concentration got higher on the support. Moreover, active site of the enzyme could be inactivated due to protein-protein interactions.14‒16 In paralellel to tour study, Rhimi et al.,17 and Alonso et al.,18 reported decreased activity and immobilization yield values of Streptococcus thermophilus β-galactosidase and Pseudomonas glutaryl acylase above a certain concentration value due to steric hindrance. In contrast, Jochems et al.,9 observed an increasing enzyme activity with the increasing enzyme concentration in contact medium when β-galactosidase was immobilized on a mixed-matrix membrane containing zirconium dioxide. Additionally maximum enzyme loading around the concentration of 600mg protein/m2 of support was calculated.
Characterization of immobilized β-Galactosidase
Effect of temperature and temperature stability: Upon immobilization properties of enzymes may change as a result of variation in the microenvironment conditions, changes in molecular conformation, and changes in the molecule charge of the adsorbed. The temperature dependence of the activities of free and immobilized β-galactosidase are shown in Figure 6. Between 40 to 50°C, immobilized enzyme obtained 87.2% to 55.6% relative activity, while free enzyme showed a significant decreased from 61.2% to 30.3%. Moreover, there was no alteration in optimum temperature of the immobilized enzyme preparation having an optimum temperature of 37°C. Unchanged optimum temperature was reported by various authors in the literature.19,20 In contrast, comparable results reported a shift of optimum temperature due to immobilization on various matrices.21‒23
Thermostability is the ability of an enzyme to resist against thermal unfolding in the absence of substrates and one of the most.24 Higher thermostability due to immobilization was shown in Figure 7. The temperature stability of free and immobilized β-galactosidase was tested at various temperatures ranging from 20 to 55°C by measuring the residual activity of enzyme after incubation for 30minutes. At 40°C, immobilized enzyme on chitosan-HAP beads retained an activity of about 41.1%, however the activity retained by the free β-galactosidase was decreased at the same temperature to 30.7%. This result suggested that immobilization onto chitosan-HAP complex showed a promising thermostability as compared to the free enzyme.
Effect of pH and pH stability: It is important to know the changes in characteristics as a result of immobilization for the construction of the reactor systems. Generally immobilization of an enzyme on a support is known to cause significant changes in the catalytic behavior of the enzyme. The activity of the immobilized enzyme can exhibit a change in optimum pH depending on the surface charge effects on local micro-environment.25 Figure 8 demonstrates the pH profiles of free and immobilized β-galactosidase. The optimum pH values resulted in a 0.5 unit shift; from pH 7.5 for the free enzyme to pH 7.0 for the immobilized one. This recorded shift in optimum pH is probably due to non-uniform distribution of hydrogen ions between the surface and the bulk solution and may lead to the shift from alkaline pH to neutral pH.8 At pH 6.0 and 7.0, free enzyme obtained 26.8% and 28.8%, respectively. Meanwhile, immobilized enzyme kept its 47.6% and 62.9% initial activity at these pH values, respectively. However, in Figure 9, it is clear that pH stability profile of free enzyme is wider in comparison with immobilized one. With the increase in pH from 6.0 to 7.5, both free and immobilized enzyme obtained very close and nearly unchanged residual activities. On the other hand, further increase in pH resulted in a sharp decrease in residual activity for immobilized enzyme, while free one preserved at least 58.6% of its original activity.26
Calculation of kinetic parameters of immobilized β-galactosidase: Kinetic parameters of the free and immobilized β-galactosidase were investigated at different concentrations of substrate ranging from 0.5-12.5 mM, and the data were processed using a typical double reciprocal Lineweaver Burk plot. As expected, kinetic parameters were significantly (p<0.05) affected after immobilization on chitosan-HAP beads. The estimated Km values, indicating the affinity of the enzyme towards the substrate, for free and immobilized β-galactosidase were found to be about 1.011mM and 9.5mM, respectively, while Vmax value was markedly decreased from 1098.9μmol ONP min−1mg-1 protein, to 454.5μmol ONP min−1mg-1 protein after immobilization onto the chitosan-HAP beads (Figure 10). Increase in the apparent Km value indicates that affinity of the enzyme to substrate is decreased after immobilization. Thus, it can be said that inactivation of the enzyme occurred during the immobilization procedure reducing the reaction rate. The increase in Km and decrease in the Vmax values of the immobilized β-galactosidases have been previously reported by other authors. Ansari et al.,27 observed an increase in Km value from 3.7mM to 3.8mM and a decrease of Vmax value from 2.7 to 1.9mM min-1 after immobilization onto glutaraldehyde modified multi walled carbon nanotubes. In another study, calculated 4 fold increase in Km value and calculated Vmax value was 8.8×10−3 μmol ONP min−1mg−1 enzyme when β-galactosidase immobilized on graphite surface.28
Operational and storage stability of immobilized β-galactosidase: Figure 11 shows the reusability of immobilized enzyme. The activity of the first batch was taken as 100%. As seen, the immobilized β-galactosidase retained 81.8% of its initial activity during consecutive eight cycles emphasizing strong linkages between enzyme and support matrix that enzyme did not leached out with repeated use.
The loss of enzymatic activity is an important aspect to investigate the industrial feasibility. Many studies showed that enzyme stability decreases during the storage (The storage stability test of free and the immobilized β-galactosidase were carried out for a period of 15days. The storage stability of immobilized β-galactosidase can be seen in Figure 12. After storage, bound β-galactosidase lost only 82.6% of its original activity within 15days.
Enhanced storage stability over 80% activity of immobilized β-galactosidase on different matrixes has been revealed earlier.29‒31
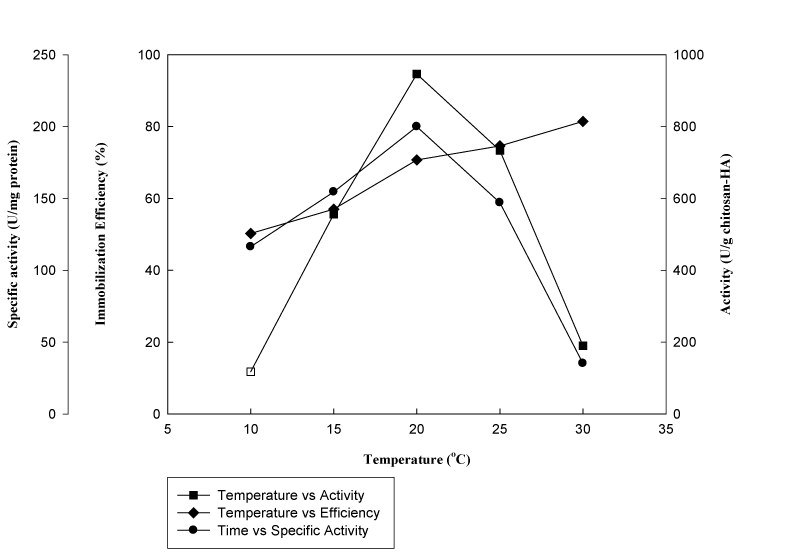
Figure 1 Effect of medium temperature on immobilization efficiency, activity and specific activity (β-galactosidase was reacted with chitosan-HAP (1.5g) in phosphate buffer (5mL, pH 6.86) at different temperatures with gentle shaking (150rpm) for 24h).
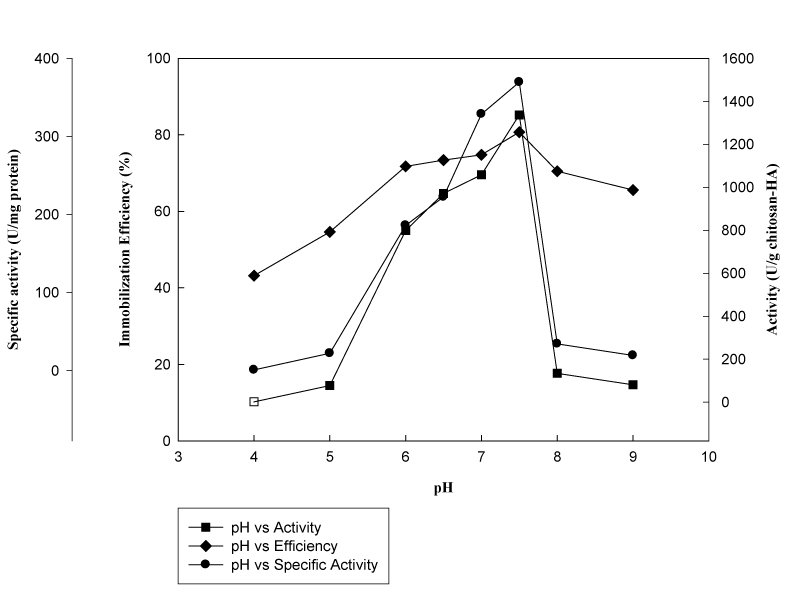
Figure 2 Effect medium pH on immobilization efficiency, activity and specific activity (β-galactosidase was reacted with chitosan-HAP (1.5g) in phosphate buffer (5mL) at different pH values with gentle shaking (150rpm) for 24h at 20°C).
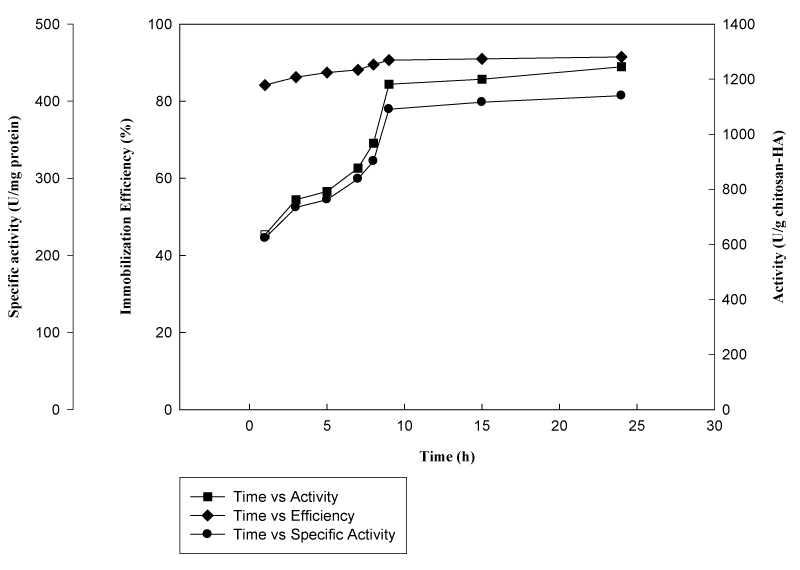
Figure 3 Effect of duration of coupling on immobilization efficiency, activity and specific activity (β-galactosidase was reacted with chitosan-HAP (1.5g) in phosphate buffer (5mL, pH 7.5) with gentle shaking (150rpm) for 24h under 20ºC temperature).

Figure 4 Effect of enzyme concentration on immobilization efficiency, activity and specific activity (β-galactosidase was reacted with chitosan-HAP (1.5g) in phosphate buffer (5mL, pH 7.5) with gentle shaking (150rpm) for 24h at 20°C).

Figure 5 Relation between amount of unbound protein and specific acivity (β-galactosidase was reacted with chitosan-HAP (1.5g) in phosphate buffer (5mL, pH 7.5) with gentle shaking (150rpm) for 24h at 20°C).
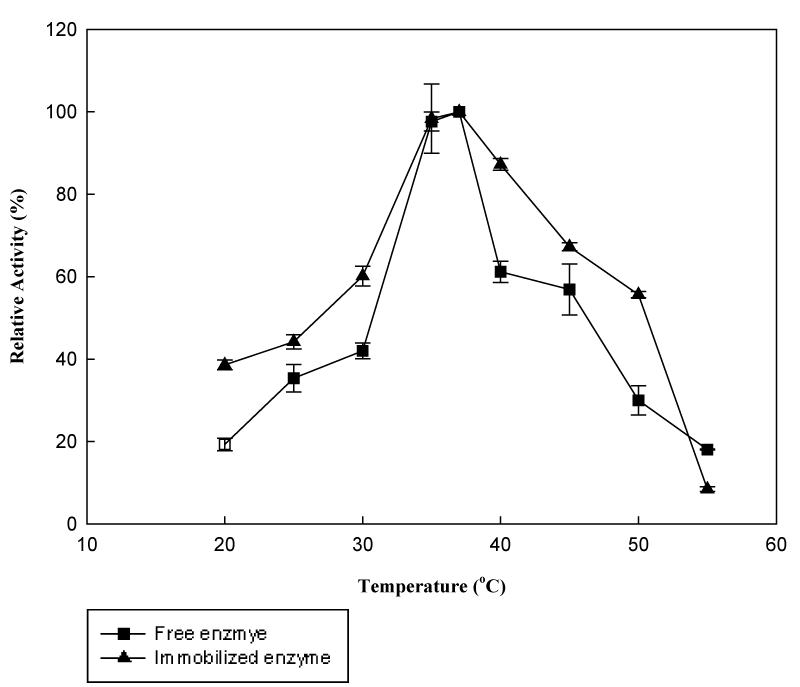
Figure 6 The effect of temperature on activity of free and immobilized β-galactosidase (Reactions were carried out at 37°C using 8.3mM ONPG in phosphate buffer at pH 6.5).
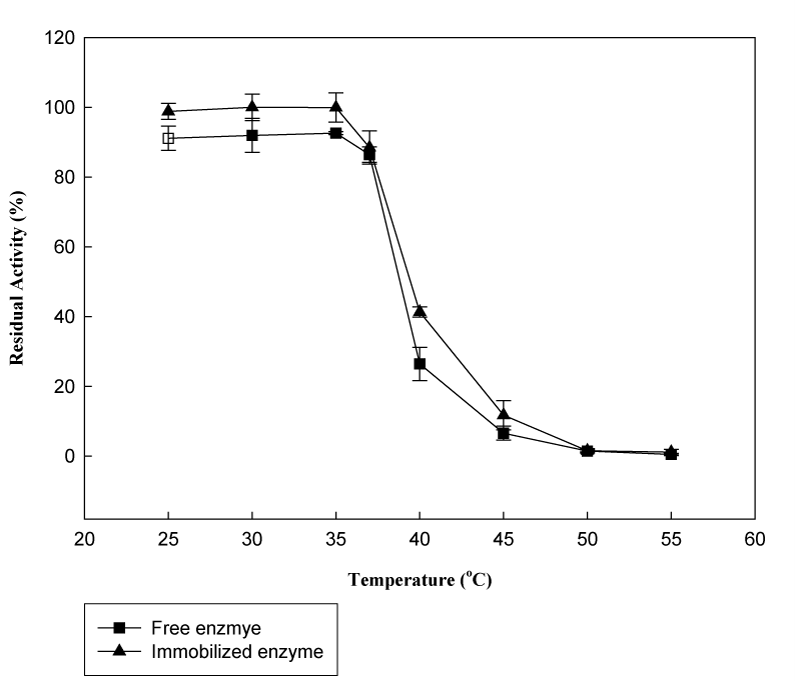
Figure 7 The thermal stability of free and immobilized β-galactosidase (Reactions were carried out at 37°C using 8.3mM ONPG in phosphate buffer at pH 6.5 after incubation at indicated temperatures).
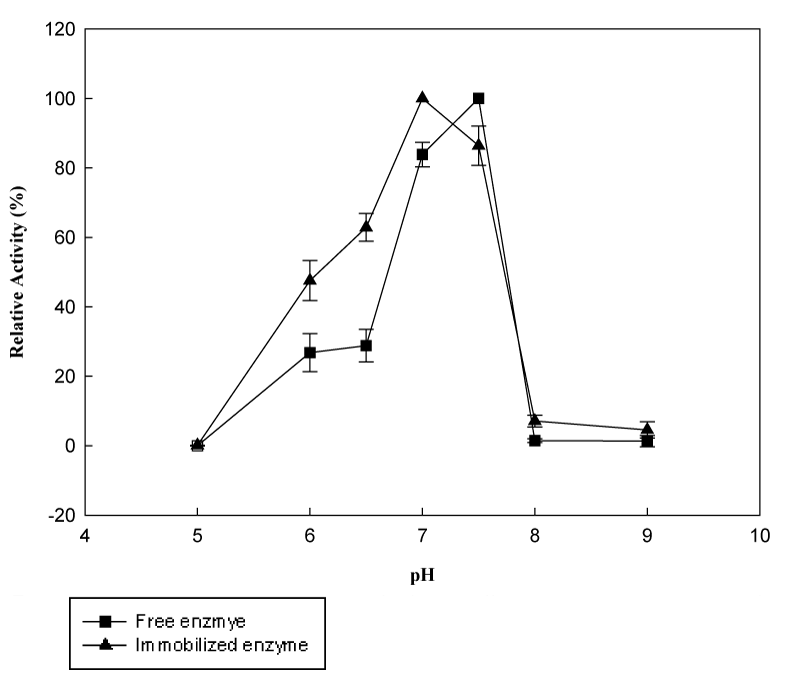
Figure 8 The effect of pH on the activity of free and immobilized β-galactosidase (Reactions were carried out at 37°C using 8.3mM ONPG in phosphate buffer at pH 6.5).
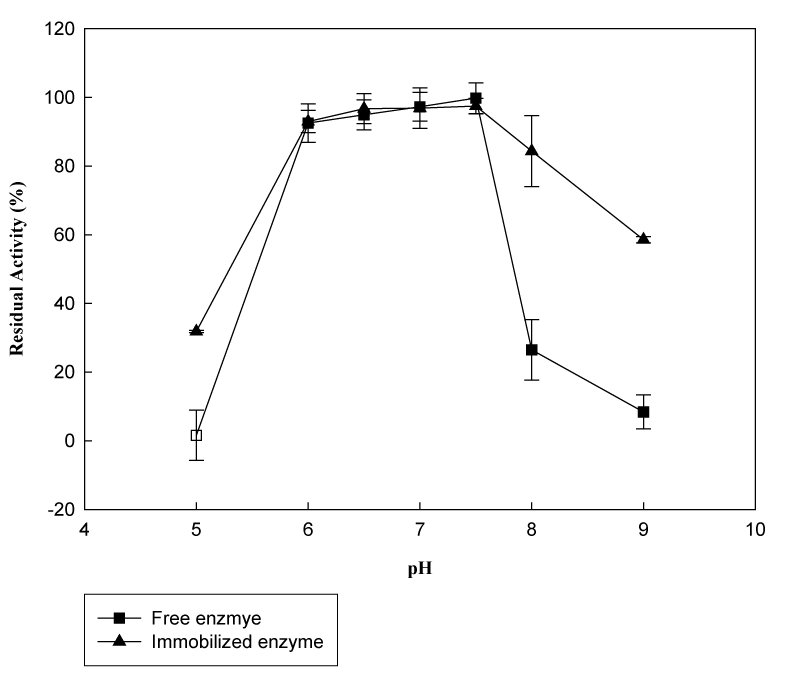
Figure 9 pH stability of free and immobilized β-galactosidase (Reactions were carried out at 37°C using 8.3mM ONPG in phosphate buffer at pH 6.5 after incubation at indicated pH values).

Figure 10 Double reciprocal plots to determine constants for ONPG hydrolysis by free and immobilized β-galactosidase.
In this work, chitosan-HAP complex was efficiently used as an immobilization matrix for β-galactosidase from Kluyveromyces lactis. The highest specific activity was recorded at the operation conditions of 20°C at pH 7.5. The activity of immobilized enzyme was inversely proportional to initial enzyme amount in the medium and using the lowest enzyme concentration of 0.23mM, the highest specific activity was obtained. For the optimization of coupling time, specific activity of 195.2Umg-1 protein was reached after 9h coupling. Immobilization onto chitosan-HAP complex altered the kinetic parameters and increased the affinity of the enzyme towards the substrate. On the other hand, immobilized enzyme exhibited remarkable broadening in pH vs activity profile. Maintaining 81.8% of its initial activity after 8 repeated uses, immobilized enzyme showed that chitosan-HAP complex can be a useful immobilization matrix for continuous conversion of lactose.
None.
Author declares that there is no conflict of interest.

©2014 Cabuk, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.