Journal of
eISSN: 2373-4310


Research Article Volume 8 Issue 3
Department of Entomology, College of Agriculture, India
Correspondence: Shashi Vemuri, Department of Entomology, College of Agriculture, Pjtsau, Rajendranagar, Hyderabad, Pin code 500 030, India
Received: November 13, 2017 | Published: June 7, 2018
Citation: Srinivasa RS, Narendra RC, Shashi V, et al. Decontamination methods utilising house hold practices for removing pesticides on field bean for food safety. J Nutr Health Food Eng. 2018;8(3):260-267. DOI: 10.15406/jnhfe.2018.08.00280
Field and laboratory experiments were conducted during Rainy season 2015 (from July to October) to study the decontamination of different pesticides on field bean. The highest removal of all insecticides from green pods of field bean were obtained from the treatment Formula 1 (4% Acetic Acid + 0.1% NaHCO3 + 1 Lemon ) ranging from 67.60 to 74.90 per cent followed by the use of 2% salt solution in reducing the residues of the pesticides chlorantraniliprole 20% SC @ 30g a.i ha-1, bifenthrin 10% EC @ 812g a.i ha-1 profenophos 50% EC @ 400g a.i ha-1, lambda cyhalothrin 5% SC @ 15.63g a.i ha-1, beta cyfluthrin (Solomon 300 OD) @ 30g a.i ha-1. Baking soda solution (0.1% ) was found more effective than 2% salt solution for the removal of fipronil 5% SC @ 500g a.i ha-1, flubendiamide 480 % SC @ 60g a.i ha-1 imidacloprid (Solomon 300 OD) @ 30g a.i ha-1. Tap water wash was the least effective method in the removal of all insecticides from green pods of field bean.
Keywords: green pods, field bean, removal of pesticides, insecticides, leguminosae
Field bean belongs to the family Leguminosae, is an important pulse cum vegetable crop in India and is cultivated extensively for its fresh tender pods, leaves and seeds and as cattle feed. In India this is grown mostly in Andhra Pradesh, Karnataka, Tamil Nadu, Kerala and Assam and the fresh and dried seeds constitute major vegetarian source of proteins in the diet and are rich in nutritive value and are rich source of carbohydrates, minerals, vitamins, such as vitamin A, vitamin C, fat and fiber. The protein content of field bean is quite high varying from 20.0 to 28.0 per cent.1 However the primary cause attributed for lower yields of field bean is due to the heavy infestation of an array of pest complex. Govindan2 recorded as many as 55 species of insects and a species of mite feeding on the crop from seedling stage to the harvest of the crop in Karnataka and considered the pod borers as important as they cause 80-100 per cent loss.3 Naik et al.,4 reported Pod borers as key impediments for the low productivity causing a loss of 54 per cent in field beans.).The major yield loss is inflicted by the pod feeders which include both the pod borers and pod bugs. Pesticide use has increased rapidly over the last two decades at the rate of 12 per cent per year and the extensive, irrational use of pesticides resulted in the presence of residues of insecticides on different edible plant parts resulting in various public health problems and ill effects on environment. The increasing amount of pesticide residues in vegetables is a major concern to the consumers as the insecticides leave residues on pods which may persist up to harvest. Effecting export and sometimes rejection of consignments. Hence, great significance has to be given to for safe consumption. As the pods are consumed as vegetable, the pest control should be with pesticides having low residues. Vegetables retain residues of cocktail of chemicals as they are applied at different stages of crop growth and often prior to harvest resulting in health hazards to the customers. Removal of these pesticide residues is of importance before consumption of vegetables, Various studies have shown that processing leads to large reduction of residues in the prepared food, particularly through washing, peeling and cooking operation.5–7 Therefore, it is essential to look for cheap and effective methods which can be implemented easily at home. Thus keeping this requirement in mind, the present studies were taken up to evaluate the effect of different household processing and lab practices to reduce pesticide residues to a safe level for human consumption.
Evaluation of decontamination methods for removal of pesticide residues
The evaluation of decontamination methods for the removal of pesticide residues was carried out by collecting zero day samples after spray from the field trial during Rainy season of 2015 from different treatments at 12kgs and made into six sets and replicated four times. One set of sample from each treatment was analyzed for initial deposits of the pesticide and remaining sets of samples of zero days from each treatment were subjected to various decontamination methods separately and these samples were analyzed for residues through validated methods. Finally the residues were calculated to know the efficiency of the various decontamination methods in the removal of pesticide residues from the field bean samples. The following decontamination / risk mitigation methods were selected for evaluation of efficiency in removal of pesticide residues from field bean.
T1 (Tap water wash):
Four liters of tap water was taken into the plastic tub of 7liters capacity and 2kg of field bean pods were dipped in the tub for 10min, followed by the tap water wash for 30sec, further the pods were kept for air drying on tissue paper for 5min.
T2 (Soaking in 2% salt solution for 10 min followed by tap water wash):
Four liters of 2% salt solution was prepared by mixing 80g of table salt in 4liters of water in plastic tub of 7liters capacity and 2kg field bean pods were dipped in the tub for 10min, followed by the tap water wash for 30sec, further the pods were kept for air drying on tissue paper for 5min, followed by analysis.
T3 (Cooking in pressure cooker for 10min):
Cooking of the 2kg field bean pods in pressure cooker for 10min, followed by the tap water wash for 30sec, further the pods were kept for air drying on tissue paper for 5min, followed by analysis.
T4 (Dipping in 0.1% sodium bicarbonate solution keep it for 10min followed by tap water wash):
Four liters of 0.1% of NaHCO3 solution was prepared by mixing of 4g of NaHCO3 in 4liters of water in plastic tub of 7liters capacity, mixture was kept for 1min and 2kg of field bean pods were dipped in the tub for 10min, followed by the tap water wash for 30sec, further the pods were kept for air drying on tissue paper for
5min, followed by analysis.
T5 (Dipping in Formula 1 (4% Acetic Acid + 0.1% NAHCO3 + 1 Lemon)
Four liters of Formula 1 was prepared by mixing 160ml of acetic acid (Vinegar), 4grams of sodium bicarbonate and 4 lemons added to 4liters of water in plastic tub of 7 liters capacity, mixture was kept for 1min and 2kg field bean pods were dipped in the tub for 10min, followed by the tap water wash for 30sec, further the pods were kept for air drying on tissue paper for 5min, followed by analysis.
Per cent removal of pesticide:
Extraction and clean –up
By using robot coupe blixer 5kg beans were homogenized and 15±0.1g sample of it was collected in 50ml centrifuge tube. Fortification was done using standard (CRM to which acetonitrile (30±0.1ml) was added. using Heidolph silent crusher .homogenization was done for 2-3minutes at 14000-15000rpm. To this material sodium chloride 3±0.1g was added and shaken gently and to separate the organic layer at 2500-3000rpm Centrifuged for 3min .16 ml of the top organic layer was collected in centrifuge tube of 50ml and the moisture content was removed adding .9±0.1g anhydrous sodium sulphate. extract (8ml) was transferred to 15ml tube having PSA sorbent (0.4±0.01g) and 1.2±0.01g anhydrous magnesium sulphate After vertexing for 30sec and centrifuging at 2500-3000rpm 2ml extract of taken into test tubes and by using turbovap evaporated to dryness and made to 1ml with n-Hexane: Acetone (9:1) for further analysis using GC ECD (Table 1).
|
Gas Chromatograph |
Gas Chromatography- AGILENT- 7890B |
|
Column |
VF-5ms Capillary Column 30m length, 0.25mm Internal Diameter, 0.25mm film thickness; 1% methyl siloxane |
|
Column Oven (0C) |
Fipronil- Initial 1800C - 2min hold - increase @ 100C /min upto 2600C - hold time 5 mins – increase @ 20C /min upto 2800C – hold for 10min. |
|
Chlorantraniliprole - Initial 1800C for 2min - increase @ 100C /min upto 2600C – hold for 15min. |
|
|
Bifenthrin - Initial 2000C for 6min - increase @ 200C /min upto 2800C – hold for 10min. |
|
|
Profenophos - Initial 1500C for 1min - increase @ 200C /min upto 2500C – hold for 9min. |
|
|
Lambda cyhalothrin - Initial 2000C for 6min - increase @ 200C /min upto 2800C – hold for 10min. |
|
|
Beta cyfluthrin - Initial 1800C - 2min hold - increase @ 100C /min upto 2600C - hold time 5min – increase @ 20C /min upto 2800C – hold for 10min. |
|
|
Detectors |
Electron Capture Detector (ECD) |
|
Detector Temperature (0C) |
300 |
|
Injector Temperature (0C) |
280 |
|
Injector Status |
Split Ratio: 1:2 |
|
Carrier Gas |
Nitrogen, Iolar II, Purity 99.999% |
|
Carrier Gas Flow (ml min-1) |
2 |
|
Make-up Flow (ml min-1) |
25 |
|
Retention time (min) |
Fipronil 8.96 |
|
Chlorantraniliprole 4.18 |
|
|
Bifenthrin 11.94 |
|
|
Profenophos 11.87 |
|
|
Lambda cyhalothrin 9.11 |
|
|
Beta cyfluthrin 19.74 |
|
|
Total run time (min) |
Fipronil 35.00 |
|
Chlorantraniliprole 25.00 |
|
|
Bifenthrin 20.00 |
|
|
Profenophos 15.00 |
|
|
Lamda cyhalothrin 20.00 |
|
|
|
Beta cyfluthrin 35.00 |
Table 1 Details of GC parameters
Gas Chromatograph- of AGILENT-7890B model was utilised for estimating the Fipronil, Chlorantraniliprole, Bifenthrin, Profenophos, Lambda cyhalothrin and Beta cyfluthrin residues utilising ECD whereas Liquid chormatograph Shimadzu make LC-30 with Mass Spectrometer (MS) mass detector was utilised for Flubendamide and Imidacloprid analysis (Table 2).
HPLC |
SHIMADZU LC-30 |
|
|
Detector |
Mass Spectrometer (MS) |
||
Column |
HPLC Column Kinetex C18 column, 2.6 micron particle size 100 length, 3mm ID |
||
Solvents in Pump A |
Water |
||
Solvents in Pump B |
Metanol |
||
Solvents Gradient Program |
Water: Methanol (5:95) mixture run for 2min |
||
Solvents Gradient rate |
0.4mlmin-1 |
||
Quantity of sample injected |
1μl |
||
Run time |
10 min |
||
Retention time |
Flubendamide-7.92min |
||
Imidacloprid- 2.29min |
|||
LC Program For flubendamide |
Time |
Methanol |
Water |
0.01 |
35 |
65 |
|
2 |
60 |
40 |
|
|
|||
4 |
80 |
20 |
|
6 |
60 |
40 |
|
8 |
35 |
65 |
|
10.01 |
Stop |
- |
|
LC Program for imidacloprid |
Time |
Methanol |
Water |
0.01 |
35 |
65 |
|
|
4 |
Stop |
- |
Table 2 Details of HPLC operating parameters
Evaluation of decontamination methods for removal of pesticide residues from Dolichos bean:
The field bean pod samples collected at zero day (2hours after application) after spray from the plots treated with fipronil 5% SC @ 500g a.i. ha-1,flubendamide 480% SC @ 60g a.i ha-1, chlorantraniliprole 20% SC @ 30g a.i. ha-1, bifenthrin 10 EC @ 812g a.i ha-1, profenophos 50% EC @ 400g a.i. ha-1, lambda cyhalothrin 5% SC @ 15.63g a.i ha-1, imidacloprid 17.8% SL @ 25g a.i. ha-1 and imidacloprid + beta cyfluthrin 300 OD @ 30g a.i.ha-1 to estimate the initial deposits and efficiency of different decontamination methods through quantification of their residues after subjecting to risk mitigation methods, and the results are presented in (Table 3) (Figures 1–9).
Insecticides |
Mean per cent removal of insecticides (%) |
|||||
|---|---|---|---|---|---|---|
Tap water |
2% salt solution |
Cooking in pressure cooker |
0.1% baking soda |
Formula 1 |
CD (5%) |
|
Fipronil 5% SC |
18.96 |
48.25 |
39.88 |
52.51 |
71.18 |
5.38 |
Flubendiamide 480% SC |
15.61 |
38.81 |
37.49 |
46.37 |
66.9 |
4.22 |
Chlorantraniliprole 20% SC |
20.28 |
52.93 |
41.5 |
51.65 |
68.28 |
2.82 |
Bifenthrin 10% EC |
29.64 |
52.29 |
37.74 |
43.11 |
67.6 |
3.96 |
Profenophos 50% EC |
11.39 |
45.12 |
29.43 |
39.88 |
72.43 |
3.26 |
Lambda cyhalothrin 5% SC |
28.77 |
51.96 |
36.88 |
43.63 |
68.9 |
4.03 |
Imidacloprid 17.8% SL |
22.74 |
61.89 |
47.67 |
56.11 |
74.9 |
7.11 |
Imidacloprid (Solomon 300% OD) |
22.38 |
61.05 |
46.87 |
62.47 |
74.29 |
3.12 |
Betacyfluthrin (Solomon 300% OD) |
28.59 |
47.06 |
38.07 |
45.78 |
69.42 |
3.19 |
Table 3 Decontamination of insecticides using different methods
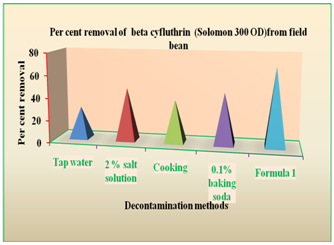
Figure 9 Per cent removal of betacyfluthrin (Solomon 300OD) residues from field bean by various decontamination methods.
Fipronil 5% SC @ 500g a.i. ha-1
Fipronil is a broad-use insecticide that belongs to the phenylpyrazole chemical family. Dipping of field bean pods in formula 1 solution for 10min followed by tap water wash for 30sec was found to be significantly effective (71.18%) in removal of insecticides than other decontamination methods. The percentage removal of fipronil residues due to various decontamination methods in descending order were, formula 1 (71.18%)> dipping in 0.1% baking soda solution (52.51%)> soaking in 2% salt solution (48.25%)> cooking in pressure cooker (39.88%)> washing with tap water (18.96%). Per cent removal of fipronil residues on field bean pods from various decontamination methods is shown in Figure1.
Flubendamide 480% SC @ 60g a.i ha-1
Flubendiamide is a novel class of insecticide belonging to thalic acid diamides family. Various decontamination methods were evaluated in order to know their efficiency in removing of flubendamide residues from field bean pods. Dipping in formula 1 solution for 10min followed by tap water wash for 30sec was found to be significantly effective (66.90%) than other treatments. The next promising treatment was dipping in 0.1% baking soda solution for 10min followed by tap water wash for 30sec (46.37%). The next best treatments were soaking in 2% salt solution followed by tap water wash for 30sec (38.81%) and cooking in pressure cooker for 10min followed by tap water wash for 30sec (37.49%) while tap water wash for 10min (15.61%) was found to be least effective in removal of flubendiamide residues from field bean pods. Per cent removal of flubendamide residues due to various decontamination methods is presented in Figure 2.
Chlorantraniliprole 20% SC @ 30 g a.i. ha-1
Chlorantraniliprole is a new class of selective insecticides belonging to anthranilic diamides family. The field bean pod samples obtained from the plots sprayed thrice with chlorantraniliprole 20% SC @ 30g a.i ha-1 were subjected to various decontamination methods. The results indicated that among the different treatments employed, dipping of field bean pods in formula 1 solution for 10min followed by tap water wash for 30sec was found to be significantly effective (68.28%) than other treatments. Soaking in 2% salt solution for 10min followed by tap water wash for 30sec (52.93%) was found to be next promising treatment, followed by dipping in 0.1% baking soda solution for 10min followed by tap water wash for 30sec (51.65%), cooking in pressure cooker for 10min followed by tap water wash for 30sec (41.50%) and tap water wash for 10min (20.28%). Per cent removal of chlorantraniliprole residues from various decontamination methods are presented in Figure 3.
Bifenthrin 10% EC @ 812g a.i ha-1
The removal of bifenthrin residues from field bean samples were significantly differed in different decontamination methods at 2hours after spraying of bifenthrin 10% EC @ 812g a.i ha-1. The results revealed that dipping in formula 1 solution for 10min followed by tap water wash for 30sec was found to be significantly effective when compared to other treatments. In this treatment residues were reduced up to 67.60 per cent. The next promising treatment was soaking in 2% salt solution for 10min followed by tap water wash for 30sec (52.29%) followed by dipping in 0.1% baking soda solution for 10min followed by tap water wash for 30sec (43.11%), cooking in pressure cooker for 10min followed by tap water wash for 30 sec (37.74%) and tap water wash for 10 min (29.64%) (Figure 4).
Profenophos 50% EC @ 400g a.i ha-1
The field bean pod samples were collected from the plots sprayed with profenophos 50% EC @ 400g a.i ha-1 were subjected to different decontamination methods at 2hours after third spray. The results revealed that dipping in formula 1 solution for 10min followed by tap water wash for 30sec was found to be significantly effective among all treatments. In this treatment residues were reduced up to 72.43 per cent. The next promising treatment was soaking in 2% salt solution for 10min followed by tap water wash for 30sec (45.12%), followed by dipping in 0.1% baking soda solution for 10min followed by tap water wash for 30sec (39.88%), cooking in pressure cooker for 10min followed by tap water wash for 30sec (29.43%) and tap water wash for 10min (11.39%) (Figure 5).
Lambda cyhalothrin 5% SC @ 15.63g a.i ha-1
The various decontamination methods were evaluated in order to know their efficiency in removing lambda cyhalothrin residues from field bean pods. The treatment with dipping in formula 1 solution for 10min followed by tap water wash for 30sec was found to be significantly effective (68.90%) than other treatments. The next promising treatment was soaking in 2% salt solution for 10min followed by tap water wash for 30sec (51.96%), followed by dipping 0.1% baking soda solution for 10min followed by tap water wash for 30sec (43.63%), cooking in pressure cooker for 10min followed by tap water wash for 30sec (36.88%) and tap water wash for 10min (28.77%) (Figure 6).
Imidacloprid 17.8% SL @ 25g a.i. ha-1
The collected field bean green pod samples were subjected to different decontamination solutions at 2hours after spraying. The results depicted that dipping in formula 1 solution for 10min followed by tap water wash for 30sec was found to be significantly effective in removing 74.90 per cent residues, than other treatments. The next promising treatment was soaking in 2% salt solution for 10min followed by tap water wash for 30sec (61.89%), followed by dipping in 0.1% baking soda solution for 10min followed by tap water wash for 30sec (56.11%), cooking in pressure cooker for 10 min followed by tap water wash for 30sec (47.67%) and tap water for 10min (22.74%) (Figure 7).
Imidacloprid + Beta cyfluthrin 300 OD @ 30g a.i. ha-1
Imidacloprid (Solomon 300 OD)
The collected field bean green pod samples were subjected to different decontamination solutions at 2hours after third spray. The results depicted that dipping in formula 1 solution for 10min followed by tap water wash for 30sec was found to be significantly effective in removing 74.29 per cent residues, than other treatments. The next promising treatment was dipping in 0.1% baking soda solution for 10min followed by tap water wash for 30sec (62.47%), followed by soaking in 2% salt solution for 10min followed by tap water wash for 30sec (61.05%), cooking in pressure cooker for 10min followed by tap water wash for 30sec (46.87%) and the least effective decontamination method was tap water for 10min (22.38%) (Figure 8).
Beta cyfluthrin (Solomon 300 OD)
The field bean pod samples collected from the plots treated with imidacloprid + beta cyfluthrin @ 30g a.i. ha-1 were subjected to various decontamination methods. The treatment with dipping in formula 1 solution for 10min followed by tap water wash for 30sec was found to be significantly effective (69.42%) than other treatments. Then next promising treatment was soaking in 2% salt solution for 10min followed by tap water wash for 30sec (47.06 %) and the treatments followed were dipping in 0.1% baking soda for 10min followed by tap water wash for 30sec (38.07 %), cooking in pressure cooker for 10min followed by tap water wash for 30sec (45.78%) and tap water wash for 10min (28.59%). (Figure 9).
To minimize dietary exposure to pesticides, it is pertinent to explore strategies that effectively help in reducing the residue content at individual level. Five simple, labour less and cost effective unit operations were imparted to field bean samples for reducing dietary consumption of pesticide residues which can be even followed in poor populace. Out of all treatments imparted each pesticide has its own treatment of reduction. In the present study, dipping in formula 1 (4% Acetic Acid + 0.1% NaHCO3 + 1 Lemon), a formulation prepared by AINP on Pesticide Residues proved to be the most efficient in removing various pesticides from field bean samples. Similar results were also reported by Radwan et al.,8 who reported that washing of hot pepper, sweet pepper and brinjal with 2% acetic acid removed pirimophos-methyl residues by 76.61, 95.74 and 94.58 per cent, respectively.
Zhang et al.,9 found that 79.8, 65.8, 74.0 and 75.0 per cent residues of chlorpyriphos, cypermethrin and chlorothalonil were removed by washing cabbage with 10% acetic acid solution for 20min, respectively. The treatment with soaking in 2% salt solution for 10min followed by tap water wash for30 sec was found to be next best decontamination method in case of chlorantraniliprole, bifenthrin, profenophos, lambda- cyhalothrin, imidacloprid and beta cyfluthrin (Solomon 300% OD formulation). The results were in agreement with the findings of Geetha10 who reported that loss of 31.47, 32.13, 46.87 and 43.78 per cent of chlorpyriphos, profenophos, cypermethrin and triazophos residues in spinach by salt water treatment for 10 min. Washing of brinjal with 2 per cent salt solution removed the 45.3, 43.0, 52.1, 49.8, 54.0, 47.9 and 76.5 per cent of dimethoate, chlorpyriphos, quinalphos, profenophos, phosalone, lambda-cyhalothrin and malathion residues, respectively.11
Washing of tomato fruits with 10% salt solution removed 90.80 and 82.40 per cent of dimethoate and profenophos residues.12 Washing of cucumbers in 2% salt solution for 10min removed residues of trichlorfon, dimethoate, dichlorovos, fenitrothian and chlorpyriphos residues by 46.30, 47.80, 70.20, 28.90 and 60.50 per cent, respectively.13
The treatment with dipping in 0.1% baking soda (NaHCO3) solution for 10min followed by tap water wash for 30sec was the next best treatment in removing residues of fipronil, flubendamide and imidacloprid (Solomon 300% OD formulation) from field beans. The results were in line with the findings of Cherukuri et al.,11 who reported that dipping with 0.1 per cent sodium bicarbonate solution in brinjal removed the 25.4, 21.5, 34.0, 29.8, 33.6, 30.4 and 61.3 per cent of dimethoate, chlorpyriphos, quinalphos, profenophos, phosalone, lambda-cyhalothrin and malathion residues, respectively.
Liang et al.,13 who reported that washing of cucumber with 2% NaHCO3 was efficient to remove the trichlorfon, dimethoate, dichlorovos, fenitrothian and chlorpyriphos residues by 73.20, 58.70, 96.40, 51.10 and 77.80 per cent, respectively, while Satpathy found that tomato fruits washed with 0.1% NaHCO3 solution removed residues of parathion, methyl parathion, malathion, fenitrothion, formothion and chlorpyriphos by 73.10, 77.40, 86.80, 57.00, 86.40 and 87.20 per cent, respectively.
The next best decontamination method was cooking in pressure cooker for 10min followed by tap water wash for 30sec. The reports of Neha et al.,14 indicated that cooking of brinjal removed the monocrotophos, quinalphos, permethrin and cypermethrin residues by 29.68, 22.84, 25.00 and 40.00 per cent, respectively, while Walia found that cooking of brinjal in water removed residues of cypermethrin 41.40 per cent.
Tap water wash for 10min was the least effective treatment and the findings of the present investigations were in agreement with the findings of Abou Arab12 who reported that washing of tomato fruits with water removed dimethoate and profenophos residues up to 18.80 and 22.17 per cent, respectively. Jayakrishnan et al.,15 reported that washing of tomato fruits with water removed lambda cyhalothrin residues by 29-30 per cent. Washing of potatoes with water removed 30-50 per cent of phosalone residues and 13.50 per cent of profenophos residues when washed with tap water. The tap water wash for 10min removed trichlorfon, dimethoate, dichlorovos, fenitrothian and chlorpyriphos residues by 36.60, 21.70, 22.60, 22.20 and 59.20 per cent in cucumber, respectively.13 Gupta et al.,16 also evaluated the dissipation and decontamination of imidacloprid and lambda - cyhalothrin residues in brinjal and found similar results Cherukuri et al.,11 reported that the loss of 30.7, 35.3, 45.6, 42, 44.1, 40.9 and 70.3 per cent of dimethoate, chlorpyriphos, quinalphos, profenophos, phosalone, lambda-cyhalothrin and malathion residues in brinjal by tap water wash while, Pallavi et al.,17 reported the loss of malathion, chlorpyriphos, quinalphos, profenophos and cypermethrin in curry leaf to an extent of 25.9, 10.8, 18.6, 21.7 and 8.2 per cent by washing with tap water for 15min. Similarly, tap water washes for 10min removed chlorpyriphos, profenophos, cypermethrin and triazophos residues by 15.37, 13.30, 19.21 and 19.88 per cent, respectively in spinach.10,18
The highest removal of all insecticides from green pods of field bean were obtained from the treatment Formula 1 ( 4% Acetic Acid + 0.1% NAHCO3 + 1 Lemon ) ranging from 67.60 to 74.90 per cent followed by 2% salt solution in chlorantraniliprole 20% SC @ 30g a.i ha-1, bifenthrin 10% EC @ 812g a.i ha-1 profenophos 50% EC @ 400g a.i ha-1, lambda cyhalothrin 5% SC @ 15.63g a.i ha-1, beta cyfluthrin (Solomon 300 OD) @ 30g a.i ha-1 while, 0.1% baking soda solution was found more effective than 2% salt solution in fipronil 5% SC @ 500g a.i ha-1, flubendiamide 480% SC @ 60g a.i ha-1 imidacloprid (Solomon 300 OD) @ 30g a.i ha-1 and least removal of all insecticides from green pods of field bean was recorded from tap water wash which ranged from 11.39 to 29.64 per cent.
None.
Author declares no conflict of interest.

©2018 Srinivasa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.