Journal of
eISSN: 2373-633X


Mini Review Volume 8 Issue 5
1Department of Radiation Oncology, Papageorgiou General Hospital, Greece
2Department of Medical Physics, Papageorgiou General Hospital, Greece
Correspondence: Efstathios Kamperis, Radiation Oncology Department, Papageorgiou General Hospital, Thessaloniki, Greece, Tel +30 2313 32 3417
Received: September 24, 2017 | Published: October 26, 2017
Citation: Kamperis E, Kodona C, Vasileios G. Oligometastatic prostate cancer: Is it real ? J Cancer Prev Curr Res. 2017;8(5):357‒359. DOI: 10.15406/jcpcr.2017.08.00295
The oligometastatic state has emerged as a transitional stage between localized and disseminated disease, potentially curable if treated with a definitive intent. Nowadays, it is being diagnosed with a rising frequency due to the advancement of imaging, MRI and functional. The prompt detection of oligometastatic prostate cancer might give the false impression of improved outcomes due to lead time bias and expose patients to treatment related toxicities. However, metastases directed therapies seem to decrease the need for subsequent palliative interventions and toxicity rates are low in high volume centers. Finally, there is some biological evidence that oligometastatic state is a genuine entity, yet we lack robust data on how to select patients for treatment intensification or how the latter affects overall survival.
There is a paraphrase often attributed to Socrates that “the beginning of wisdom is the definition of terms”. Oligometastasis is a compound word formed from oligo which means few or scant and metastases. The discussion, therefore, centers on prostate cancer patients with “a limited number of metastases”. Although there is no consensus many studies use ≤5 as a cut-off point.1,2 whereas others use ≤3 with additional constraints on the location of metastatic foci.3
The term oligometastasis (o-mets) was first coined by Hellman & Weichselbaum.4 The data available at the time suggested a stepwise progression of cancer from localized disease to an intermediate state of malignancy and then to widespread metastases. The practical implication of this suggested paradigm shift was that some patients could potentially be cured with definitive local therapies or achieve long-term remissions that could not be feasible with systemic therapies.
Although most of prostate cancer patients have localized disease, 12% have regional disease and 4% have distant metastatic spread at the time of diagnosis.1. For these patients, there are many treatment options, given either alone or combined: androgen deprivation therapy (ADT), chemotherapy and local ablative therapies. Current guidelines.5 do not include recommendations for local treatments minus the case of lymph node positive disease, following the publication of STAMPEDE’s trial results. In this study patients with N+M0 disease in the control arm that received radiotherapy, in a nonrandomized manner, had a better 2-year failure-free survival.6
In Figure 1 the natural history of a patient with prostate cancer is plotted against time.7 The horizontal axis represents time and the vertical axis is PSA concentration. Besides PSA, we could plot the disease course against some other quantifiable variable e.g. symptomatic or radiographic response. The blue circles represent the opportunities to alter the disease course via a metastasis-directed therapy (MDT) at the time of maximal response. The area of the circles is qualitatively proportional to that probability. As the disease evolves, the chances are getting smaller and smaller and at some point, they disappear. The case of an intermediate clinical entity, potentially curable with local ablative techniques has been described by some as wishful thinking.8 We do have though some supporting evidence that oligometastatic state represents a true intermediate biological state.9 This state is explained on the basis of microRNA-mediated attenuation of prometastatic epithelial plasticity programs, such as the epithelial-mesenchymal transition.10 These results demonstrate a biological basis for o-mets and a potential for using microRNA expression to identify patients most likely to remain oligometastatic after MDT.
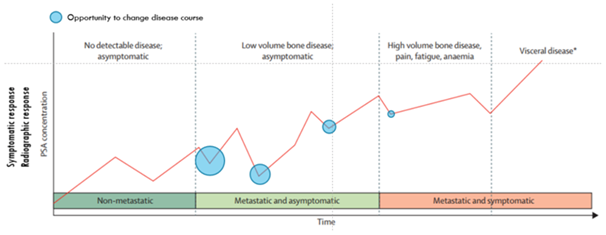
Figure 1 Natural history of a patient with prostate cancer is plotted against time.7
Figure 2 depicts the natural history of a cancer from time zero to death. With rigorous imaging surveillance, we identify oligometastases before they become symptomatic and we introduce the so-called lead time bias. Patients end up receiving therapy at an earlier stage of their disease evolution, potentially being exposed to treatment side effects. At the same time survival appears to increase, although in reality remains unchanged and the overall morbidity is lengthened. Nevertheless, we do not think that the results we observe in various series could be justified solely on the basis of lead time bias. Also, ≥ grade 3 treatment side effects of SABRT in experienced centers are particularly low.11
We are thus brought to the fundamental question on how to select patients for local treatment intensification. Given that we currently lack high quality prospectively validated predictive factors, we must rely on the clinical, radiographic, scintigraphic and serologic phenotype of the disease. Such parameters include the interval between radical local treatment and the development of metastases.12 Also, the duration and depth of response to hormonal manipulation are known to predict overall outcomes. The PSA kinetics along with the number of metastases, up to five.2 or in some series up to three3, are surrogate markers for tumor burden and biological aggressiveness. Finally, the metastatic site itself needs to be considered. Lymph node and bone metastases herald a better prognosis than visceral mets and even between the group of patients with visceral disease those with lung mets do better than those with liver.13,14 Besides, patients with visceral metastatic foci are under-represented in most of the published retrospective studies.11 Hence, any extrapolation of the conclusions from these studies to patients with visceral disease is not prudent. Obviously, there is a whole spectrum of patients in between these two extremes and we lack the tools to stratify them.
Unfortunately, only a fraction of oligometastatic patients will experience long-term disease control with aggressive treatment of limited metastatic foci. Identification of this subset may be enhanced by using molecular selection criteria, which could enrich the therapeutic benefit of MDT, while redirecting patients unlikely to benefit from surgery or radiotherapy to systemic treatments. Similarly, patients with metastatic disease that at first presentation would appear not amenable to local treatment but exhibit an oligometastatic genotype might benefit from a combined aggressive local and systemic approach.9
Of particular interest is the report of the latest advanced prostate cancer consensus conference (APCC).3. The panel considered the following cases. In the first clinical scenario, a patient is diagnosed with synchronous o-mets and an untreated primary. Most physicians would choose a local ablative therapy along with long term ADT with or without upfront docetaxel. We would probably opt for local ablative plus short-term ADT, potentially sparing the patient from the side effects of long term hormonal blockage. In the second clinical scenario, a patient presents with o-mets after having been treated with a local treatment of the primary tumor. Most would recommend lifelong ADT with or without docetaxel. Depending on the clinical and radiographic phenotype of the disease, we would at least consider the possibility of a local ablative therapy plus short-term ADT with or without docetaxel. In the last clinical scenario, a patient with a rising PSA while he is on ADT is diagnosed with oligometastases. We agree that this might not even comprise a meaningful clinical entity and a continuation of ADT plus systemic therapy would represent the most relevant course of treatment. Perhaps we could contemplate a local ablative therapy in patients with favorable prognostic factors.
Fortunately, there are quite a few ongoing trials that will soon shed some light on this highly debated topic. For example, the ORIOLE trial randomizes patients with up to 3 mets and an hormone-naive disease to observation or SBRT.11 Similarly, the STOMP trial from Belgium will examine surveillance or MDT for oligometastatic prostate cancer recurrence; it’s a phase 2 randomized trial.15 Of particular interest is the OLIGOPELVIS trial from GETUG group, a phase 2 multicenter trial of combined salvage RT and hormone therapy in oligometastatic pelvic node relapses.16
CA184-043 was a very important multicenter, randomized, double-blind, phase III clinical trial comparing ipilimumab versus placebo after radiotherapy with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy.17 Patients received radiotherapy to bone metastases (8 Gy) and then randomized to either ipilimumab or placebo. Although there was no difference in overall survival (primary endpoint), in a post-hoc subgroup analysis a favorable group of patients emerged consisting of those with no visceral compromise, Hb≥11 g/dl and ALP≤1.5 ULN. In this group, despite patients been heavily pretreated, overall survival was 22.7 months with ipilimumab versus 15.8 months with placebo. The reason why we think this trial is important is because data are emerging on the interplay between high dose radiotherapy and immunotherapy.18
There is an intermediate state in prostate cancer between localized and widespread disease with a distinct biological profile, probably underestimated thus undertreated. There is an urgent need for prospective clinical trials to validate patient selection criteria and establish the standard of care. Until then, patients with late recurrences, deep and prolonged response to ADT, one to three number of metastases and lack of visceral disease represent good candidates for treatment intensification in the context of metastasis directed therapies.
There is no conflict of interest.
None.
None.

©2017 Kamperis, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.