Journal of
eISSN: 2373-633X


Research Article Volume 14 Issue 1
1Leader researcher, Oncogenomics Lab, NN Blokhin Cancer Research Center (NN Blokhin CRC), Russia
2Minor researchers, Oncogenomics Lab, NN Blokhin CRC, Russia
3Researcher, Lab for Tumor experimental diagnostics and therapy, NN Blokhin CRC, Russia
4Head of Lab for Tumor experimental diagnostics and therapy, NN Blokhin CRC, Russia
5Head of Department for Peptide Immunogenes, State Sci Center “Immunology Institute”, Russia
Correspondence: Dr. Anna A Lushnikova, NN Blokhin Cancer Research Center, 115478 Kashirskoye shosse-24, Moscow, Russia, Tel +7 499 323 5666
Received: February 06, 2023 | Published: February 16, 2023
Citation: Lushnikova AA, Onyan AV, Kovtun OG, et al. Novel approaches for overcoming of tumor drug resistance by polyvalent cationic peptides. J Cancer Prev Curr Res. 2023;14(1):24-27. DOI: 10.15406/jcpcr.2023.14.00513
Background: Acquired resistance to various drugs is a key challenge for anticancer therapy. Earlier we have revealed a selective apoptosis of tumor cells induced by polyvalent cationic peptides (CPs) both in vitro and in vivo by inactivation their cell targets – multifunctional chaperone proteins nucleolin (NCL) and nucleophosmin (NPM). Here we applied Arg/Lys-enriched CPs as well as CPs, conjugated with Doxorubicin (Dox) to induce cell death by nucleolar stress mechanisms both in Dox- sensitive breast cancer (BC) cells and in Dox-resistant ones. Molecular interactions between CP as ligand and cellular targets using computer modeling by docking have been done. Then, CP potential for anticancer therapy of various malignant tumors is discussed.
Objective: An analysis of cell survival in breast cancer (BC) sublines that are sensitive or resistant to Doxorubicin (Dox) by cationic peptides (CPs) and conjugate CP +Dox.
Results: The data indicate that Arg/Lys enriched CPs might be perspective agents for inducing BC cell apoptosis and for transport Dox or other drugs in tumor cells to overcome drug resistance.
Conclusion: Molecular docking is effective tool for modeling and evaluating of interactions and competitive binding of CPs to their cellular targets. This approach is also useful for design novel peptides with high anticancer properties and low toxicity for patients.
Keywords: breast cancer (BC) cell sublines, acquired resistance to Doxorubicin (Dox), chaperone proteins NC and NPM, cationic peptides (CPs), Dox-resistant BC cell apoptosis
Development of molecular-targeted drugs and analysis of mechanisms for antitumor activity is an actual problem for modern oncology. As a rule, many of the metastatic tumors of different localization are not complete recovery and effective therapy of such malignant tumors remains very difficult problem. So an analysis of molecular mechanisms of the selective antitumor activity of cationic peptides (CPs) is actual. These Arg/Lys –enriched CPs have rather low molecular weight (about 2 000 Da).and low toxicity for normal cells.1 Analysis of the cytotoxicity for some CPs on the model cell lines of breast cancer (BC) has revealed significantly higher expression of shaperone proteins NCL and NPM in analyzed tumors than in morphologically normal cells and tissues. It explains the selective cytotoxicity of CPs, which are considered as a ligands for nucleolin, which is highly expressed on membranes of tumor cells, and nucleophosmin.2 These nucleolar proteins regulate the activity of most cellular enzymes, ribosomal biogenesis, cell spindle assembly, chromatin remodeling , transcription and translation, and other vital processes. Therefore, the protein inactivation leads to cell death. The proposed mechanism includes a binding of cell surface NCL molecula by cationic peptides, dislocation nucleophosmin from the nucleus to the cytoplasm, the release of suppressor p53 and subsequent apoptosis.3 These results justify the strategy for obtaining anticancer drugs on the basis of cationic peptides for overcoming acquired drug resistance.
Understanding of molecular interactions can help speed drug design, while reducing costs. Computer technologies might be critical in finding molecules that bind to disease-causing proteins, including rather small peptide ligands, as well as evaluating the relationship between structure and functions of these molecules. Characterization of binding properties can lead to early prediction of drug candidate efficacy.4 When a suitable candidate is identified, a strategy is used for detailed kinetic and molecular dynamics analysis, target and binding specificity, as well as immunogenicity and stability analysis to ensure the release of effective pro-drug with anticancer activity, low toxicity and side-effects for patients, and other optimal characteristics.
Original line of BC cells HBL-100 was obtained at Cytology Institute of Russian Academy of Sciences (St-Petersburg). Resistant BC cell subline has been obtained after 2 week incubation HBL-100 cells in complete DMEM medium supplied with 10% embryonic bovine serum and containing 0.3 mkM Dox, followed by cell selection.5 Two CPs - AM-2 and NC 783 (Table 1) were synthesized by solid phase method using F moc-protective strategy Dox was conjugated with .CP KK 13-Dox (Figure 1).Cytotoxicity of three CPs and cell viability was estimated by standard MTT-assay using control human skin fibroblast line Wi-38. Both HBL-100 and HBL-100/Dox sublines were cultured 72 h in 96-well plates in presence or absence (control) of CP solution in growth medium, 0.5-4.0 μg/ml. Then MTT aliquots of 5μg/ml were supplied per each well followed by incubation at 37ºC for 2-3 h. The reaction was monitored at A480 nm. For western bloting, monoclonal antibodies to NCL/NPM/P53 were used according to the manufacturer's recommendations (Abcam/DIAEM). Concentrations of proteins resolved from BC cell lysates were determined by the Bradford method (BioRad). Fluorescently labeled monoclonal antibodies to NCL, NPM, and b-actin conjugated with horseradish peroxidase and the first mouse IgG antibodies (Sigma, USA) were used after protein immunoprecipitation, 7.5% SDS-PAGE electrophoresis and transfer to a nitrocellulose filter (Figure 1).
|
# |
Formula |
Molecular weight/charge |
Effective concentration (μg/ml) |
Proportion of alive HBL-100 cells (min-max) in MTT (%) |
|
1 |
AM-2/Mir-KRPARPAR-NH2 |
1391/4+ |
≥0.50 |
4-6/20-25 |
|
2 |
NC783/KR2G3KL2KL3KL3KL2KC |
2505/9+ |
≥0.85 |
4-5/38-40 |
Table 1 Some physical and chemical properties of the peptides
For molecular docking: we used citemap program Maestro 11 with maximal docking box size of 36Ǻ and this area included the entire target proteins. Thus, one can calculate the potential grid throughout the protein using glide scores. The ligand (CPs) preparation procedure has also included protonation, pH = 7.0. Moreover, we have done at list 4 conformers for each ligand.6
The high cytotoxicity of the tested CPs was revealed both for Dox–sensitive, and Dox-resistant BC cells (IС50=1μg/ml) (Figure 2), while control fibroblast survival did not significantly change (not shown).
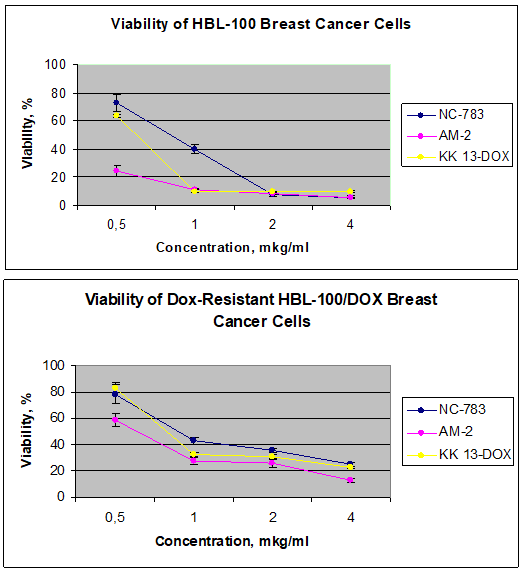
Figure 2 Viability of Dox-sensitive (A) Dox-resistant (B) BC cell sublines after 72h-incubation in presence of CPs NC-783, AM-2 and KK13-Dox with various delutions.
Apoptosis of HBL100 cells with activation of caspases 3, 8, 9 (Table 2), and HBL100/Dox cells with activation of caspases 9, 2 and p53-signaling cascade was observed by FITC labeled caspase kit and cytometry.
Activation |
Caspase-3 |
Caspase-8 |
Caspase-9 |
AM-2 |
39.30% |
47.70% |
87.40% |
KK 13-Dox |
28.70% |
37.80% |
97.00% |
NC-783 |
43.60% |
27.90% |
61.00% |
Control |
19.30% |
16.20% |
28.00% |
Table 2 Caspase 3,8, 9 activation, induced by CPs in BC HBL-100 Dox-sensitive cells
The main criterion for assessing the survival of cells after incubation with peptides is over expression of NCL molecular on the surface of these cells and in the cell nuclei. Nucleolin, NCL and nucleophosmin, NPM are highly expressed in the most of solid tumors, including BC cells (Figure 3). Nucleolin is also expressed at cell surface membrane of tumor cells and it is considered now as a therapeutic target for different cancers.2
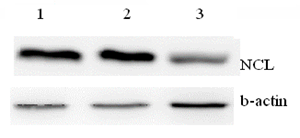
Figure 3 NCL expression levels in HBL-100 (1), HBL-100/Dox (2) BC cells and Wi-38 fibroblasts.3
The interaction of the chaperone protein NCL, which also has own kinase activity, with various cellular targets, including growth factor receptors, leads to activation of cellular signaling and uncontrolled proliferation. Inhibition of functional activities of NCL causes cell death by nucleolar stress and apoptosis (Figure 4). According our data, BC cell apoptosis was accompanied by an increase of the frequency of double-stranded DNA breaks, degradation of chromatin and mitotic spindle, migration of NPM from the nucleus and activation of the p53 suppressor protein.3,5
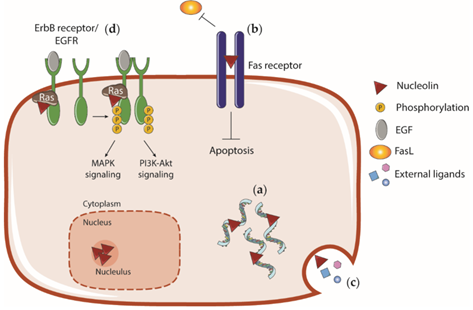
Figure 4 Some mechanisms of CP cytotoxicity for cancer cells. Inhibition of the functional activity of NCL by CPs leads to disruption of a number of cellular functions and signaling pathways (a-d) with the subsequent development of apoptosis.
Analysis by molecular docking between CP and NPM, CP and NCL, CP and pGP protein; CP-Dox and pGP revealed significantly more energetically advantageous binding (scores modulo) between CP and NCL. In particular, the binding of pGP to CP KK-13-Dox is characterized by a maximum estimated function = – 6,202. Since the interaction of this CP with the receptor NCL is energetically more advantageous (glide score= –10.78 ), the transport of CP molecules is supported into both Dox-sensitive and Dox-resistant BC cells with their subsequent death, mainly due to apoptosis. Thus, a release of CP KK-13-Dox out of these cells is low. This competitive binding of CP, including conjugate with doxorubicin CP KK 13-Dox (Figure 5), overcomes Dox release caused by hyperactivation of pGP in HBL Dox-resistant cells. Then apoptosis is induced in resistant cell subline by inactivating NCL molecules, which are hyperexpressed on the surface of malignant cells with subsequent inhibition of nucleophosmin (NPM) and development of stress-induced apoptosis.
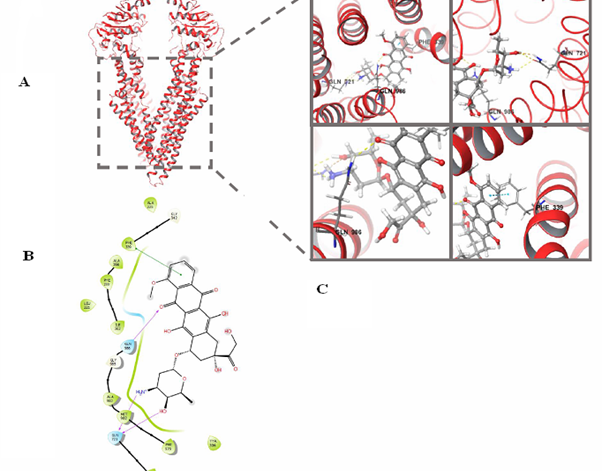
Figure 5 The structure of the pGP transporter protein and the scheme of its interaction between CP, conjugated with Doxorubicin (KK 13-Dox).
Moreover, analysis of intermolecular interactions between CP, NCL and NPM using pairwise docking and 3D modeling revealed high evaluation functions (glide scores) and binding of peptide ligands to certain amino acid residues of NCL and NPM (Figures 6 & 7). The possibility of CP interaction with NCL is also confirmed by the results obtained using immunoprecipitation and analysis of the Unified Human Interactome (UniH1) database.
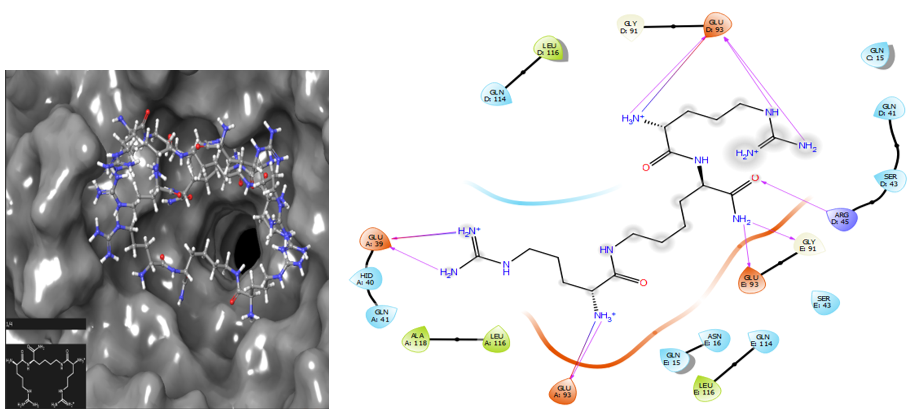
Figure 6 Paired molecular docking of NPM–NC 810, glide score = – 10.786, ligand – CP is localized in the active center of the protein, significantly inactivating its functioning. Diagram shows the formation of hydrogen bonds between the residues Gly 39, 93, Arg45, Gly91 within the active center of NPM.
Recently, the interest in use of peptides and their derivates in oncology has significantly grown.7 Analysis of molecular mechanisms of the selective anticancer activity of cationic peptides with low molecular weight and low toxicity for normal cells has found the preferred cellular targets of these CPs –multifunctional chaperone proteins NCL and NPM. The expression of these proteins play an important role in key cellular processes, it is significantly higher in analyzed tumors than in morphologically normal cells and tissues.2,5 It is a basis for the selective cytotoxicity of the CPs, which are considered as a ligands for nucleolin, which is highly expressed on the surface of tumor cells. These chaperone proteins have anticancer, antiviral and immunomodulatory activities. Due to specific molecular structure and functions, they regulate the most cellular enzymes, ribosomal biogenesis, cell spindle assembly, chromatin remodeling, transcription and translation, and other vital processes. Therefore, the protein inactivation leads to cell death. Its proposed mechanism is a binding of cell surface NCL by cationic peptides, dislocation NPM molecular from the nucleus to the cytoplasm, the release of suppressor p53 and subsequent apoptosis.3 It would allow to develop fundamental algorithm for the applications of some cationic peptides as anticancer agents with high selective toxicity to tumors, regardless of cancer origin and localization. The expected results justify the strategy for obtaining targeted drugs on the basis of cationic peptides taking into account the expression of their cellular targets, and a certain tactics for clinical trials.
The study of chaperone proteins NCL/NPM as potential targets for targeted therapy of Dox-sensitive and Dox-resistant BC cells by CPs has revealed: 1. Inhibition of the functions of nucleolin/NCL and nucleophosmin/NPM nuclear proteins by CPs with subsequent death of tumor cells, including drug-resistant BC cells. 2. Cationic peptides enriched with Arg/Lys have selective cytotoxicity against tumor cells of various origins with a high level of expression of chaperone proteins NCL/NPM and glycosylated NCL on the membrane of these cells. 3. Moreover, the tested CPs might be used as carriers for targeted delivery of compounds (chemotherapy drugs, miRNA, etc.) to the tumor. Since this CPs are nucleotropic and serve as ligands for NCL molecula expressed on the surface of tumor cells.
The authors are grateful to Dr. Natalia Moiseeva for HBL-100 cell culture gift.
The authors declare that there no conflict of interest.

©2023 Lushnikova, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.