eISSN: 2379-6367


Mini Review Volume 6 Issue 5
1IFBV-BELHERB, Luxembourg
2University of Kolwezi, Democratic Republic of Congo
Correspondence: Pierre Lutgen, IFBV-BELHERB, BP 98 L-6905, Niederanven, Luxembourg
Received: September 17, 2018 | Published: October 15, 2018
Citation: Lutgen P, Munyangi J. Platelets, eryptosis, amiodarone, aspirin, Artemisia. Pharm Pharmacol Int J. 2018;6(5):377-381. DOI: 10.15406/ppij.2018.06.00205
Platelets play an important role in malaria. They contribute to the eryptosis of Plasmodium infected erythrocytes and to the phagocytosis of merozoites. Some herbs or drugs like Artemisia, amiodarone, aspirin may interfere with this process, positively or negatively.
Thrombocytopenia
Thrombocytopenia is a common finding in malaria. Median platelet counts are lower among severe cases than in mild cases, and in children who died than among those who recovered. Infected erythrocytes may adhere to platelets, and the clumps of infected erythrocytes and platelets are associated with severe disease.1 The major impact of malaria is on thrombocytes. It is more frequent than anaemia and can be used as a predictor of severity of malaria. A review paper from Morocco studied the main hematological disturbances due to malaria. Thrombocytopenia was found in 90% of cases, lymphocytopenia in 58% and anemia only in 23% of cases. Similar hematological changers were found by several authors.2-12 Graphical representation shows it clearly (Figure 1)(Figure 2).
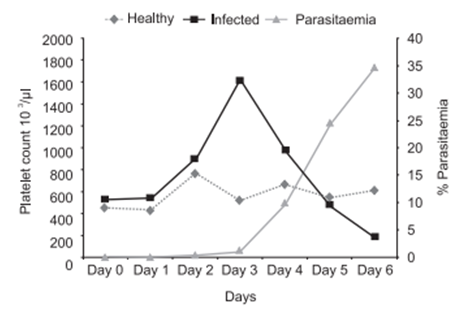
Figure 1 Platelet count fluctuations associated with P. berghei rodent malaria model.13

Figure 2 Hematological changes in P. Falciparum and P. Vivax malaria.14
But the role of these dramatic changes in platelet count is not well understood and the opinions are controversial. Some claim that platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Platelets bind directly with infected erythrocytes, particularly mature stages. They bind less to uninfected erythrocytes in the bloodstream. Platelet-erythrocyte complexes comprise a major proportion of the total platelet pool in malaria patients and may therefore contribute considerably to malarial thrombocytopenia. Other authors describe platelets as covercytes with pseudopods, rather than phagocytes.15-18 A quite opposite thesis is presented by another research group, stating that thrombocytopenia correlates with the development of severe falciparum malaria.19 Experiments in mice showed that animals genetically deficient in platelets were significantly more susceptible to death from Plasmodium chabaudi infection than were isogenic non–platelet-deficient mice. Similarly, aspirin-treated mice were more susceptible to death from P. chabaudi infection than were placebo-treated animals.20
Eryptosis
Platelets probably play a key role in the eryptosis of Plasmodium infected erythrocytes. Similar to apoptosis of nucleated cells, eryptosis or suicidal erythrocyte death is a physiological mechanism eliminating defective erythrocytes. It is characterized by erythrocyte shrinkage and cell membrane scrambling. Eryptosis is triggered by a wide variety of xenobiotics, others may inhibit it. Eryptosis stimulating xenobiotics may accelerate removal of Plasmodium infected erythrocytes and thus favorably influence the clinical course of malaria. Eryptosis, in malaria, is not restricted to parasitized cells. Eryptosis participates in the innate defense through restriction of intracellular pathogens propagation. In an in vitro culture it was shown that while parasites infected and developed normally in control non-eryptotic erythrocytes, cultures developed with eryptotic erythrocytes had a marked decrease in parasitemia. A great number of free merozoites remained in eryptotic cultures, indicating that these cells were not susceptible to invasion and consequently controlled parasite propagation.
Malaria parasites are dependent on glucose as a nutrient source. As Plasmodia have no capacity to store energy in the form of glycogen, they only rely on an exogenous supply of glucose. The metabolism of the infected erythrocyte uses up to 75 times more glucose than uninfected erythrocytes. Glucose is vital for Plasmodium and the erythrocyte where it took refuge. Glucose metabolism represents a critical physiological program that not only provides energy to the cell and the parasite it contains to support proliferation, but also directly modulates signaling pathways of cell death. It is likely that a parasite infected erythrocyte at a given moment has exhausted entirely the exogenous supply of glucose. Hypoglycemia is a common problem in malaria. Platelet counts fall in association with hypoglycemia. A study from Tübingen has shown that platelets adhere to glucose depleted suicidal erythrocytes under flow conditions. This will eliminate eryptotic erythrocytes and the merozoites they contain but may also foster thrombo-occlusion and cerebral malaria.21-26
Amiodarone
The malaria parasite faces a dilemma. It depends on the opening of ion channels in the erythrocyte cell membrane, as they allow the uptake of nutrients, but also Ca⁺⁺. Ca⁺⁺ however stimulates eryptosis and simultaneous death of the parasite. The intraerythrocytic parasite deals with this dilemma by delaying the execution of eryptosis by mechanisms well described in the following paper.27 Erythrocytes with haemoglobulinopathies or G6PD-deficiency are highly prone to enter eryptosis and this protects the human carrier. Thus, manoeuvers accelerating eryptosis may result in premature eryptosis. Amiodarone which is used to control irregular heartbeat also has a protective effect in malaria. It triggers eryptosis and the clearance of malaria infected erythrocytes. In Plasmodium berghei infected mice amiodarone injections increased the survival. It is interesting to note that amiodarone is also active against other tropical diseases like Chagas or Leishmaniasis, both in vitro and in vivo, probably by disrupting the parasites' Ca⁺⁺ homeostasis.28-31
Amiodarone induces hepatocyte microvascular lipid accumulation, and a significant decrease in serum triglycerides and glucose. One of the side effects of amiodarone is the disruption of the hepatic lipid homeostasis. Amiodarone generates lipid containing vacuoles within the hepatocytes.32 Very low density lipoproteins (VLDL), are rapidly cleared from plasma and enter hepatocytes. It has been suggested that remnant lipoproteins are initially captured in the space of Disse and that their subsequent internalization into hepatocytes is mediated by members of the LDL-receptor gene family. Similarly to lipoprotein remnants, malaria sporozoites are removed from the blood circulation by the liver within minutes after injection by Anopheles mosquitoes. The sporozoite's surface is covered by the circumsporozoite protein (CSP), and its region II-plus has. Sporozoites, remnant lipoproteins are cleared from the blood compete in vitro and in vivo for binding sites on liver cells.33-35 In a previous paper, we described the prophylactic effects on malaria of a ketogenic, high fat diet.36,37
Plasmodium needs cholesterol, especially HDL, for its development. After a malaria infection the cholesterol load in the human body diminishes. This is confirmed by many studies and a study from India. The interesting feature of the latter is that they describe accurately that this decrease in total cholesterol has nothing to do with fever per se, but is specifically related to the Plasmodium infection. Very strange is also their finding that only HDL and LDL decrease, but that VLDL sharply increases. Previous studies on the lipid profile in malaria had not noticed this change because they only measured LDL and HDL and ignored VLDL. This VLDL increase is poorly understood. It is possible that at the release of the sporozoites from the liver at the same time VLDL is released. The fact that this increase is noticed on day one of the erythrocyte invasion by merozoites pleads in favor of this hypothesis.38
Immunoglobulins
In malaria endemic areas the population carries a higher level of immunoglobulin antibodies. IgE in association with monocytes or platelets may give rise to reactions that are protective and/or pathogenic. Most children and adults living in areas where the endemicity of Plasmodium falciparum malaria is high, have significantly elevated levels of both total IgE antibodies and specific antimalarial IgE bodies in blood. IgE containing immune complexes are known to give rise to monocyte activation via the NO transduction pathway.39 Intravenous administration of human immunoglobulin increases platelet counts. The effect is dose dependent and permits to correct thrombocytopenia. The receptor for immunoglobulin on human platelets has been identified.40,41 The higher number of IgE and IgG antibodies in immune persons is positively correlated with merozoite phagocytosis by monocytes. Platelets opzonised by IgG activate monocytes to regulatory cells. This activation of monocytes is also seen in leishmaniasis. In mice deficient in platelets the recruitment of monocytes is much slower.42-44 Monocytes do ingest merozoites of Plasmodium falciparum, but rarely phagocytose parasitized or non-parasitized erythrocytes. Already in 1981, a French study showed that the monocytes from hyperimmune subjects living continuously in endemic areas were significantly more efficient in the ingestion of merozoites than were those obtained from noninfected subjects or immune individuals who had been away from endemic areas for more than a year. The authors suggest that the role of antibodies can be highly suspected. Similar effects have been observed in rats for the opsonization by antibodies of Plasmodium berghei merozoites (Figure 3).45

Figure 3 Phagocytosis of P. falcipuram merozoites by monocytes from normal and malarial subjects.46
Abbreviations: HI, hyper-immune; I, immune; PA, primary attack; N, normal control.
For IgM the situation is controversial. IgM, the least studied and most enigmatic of the vertebrate Igs, was recently shown to form an intimate relationship with the malaria parasite Plasmodium falciparum. This represents a hitherto unknown immunoevasive mechanism. It was first described in 1972. IgM increases during the 10 first days of the infection and then reverts to the initial values on day 28. Understanding how infected erythrocytes manipulate IgM may also lead to the development of improved therapies.47-49
Aspirin
Aspirin (acetylsalicylic acid) is sold over the counter in Africa and appears to be the first line treatment when children or adults have fever. Chronic salicylate poisoning by overdoses is common. Many children brought to a hospital in Africa have a high load of salicylic acid in their blood. Paracetamol is also of common use. In all children admitted to Kilifi District Hospital between July and September 1994, who had a positive blood film for Plasmodium falciparum, and one or more of coma, prostration, or respiratory distress salicylate concentrations were measured. Data were available for 143 children with initial primary diagnoses of severe malaria. 129 had detectable (>lmg/dL) salicylate. Six of these had salicylate concentrations of 20mg/dL or higher. All six had neurological impairment and metabolic acidosis and four were, or became, hypoglycaemic. 21 percent of the mothers gave a dose of aspirin higher than the manufacturer's recommended maximum. These cases suggest that in some children salicylate poisoning may cause or contribute to the development of metabolic acidosis and hypoglycaemia, complications of severe malaria associated with high mortality.50 Aspirin, like other platelet antagonists, abrogates the antiparasitic activity of human platelets. Salicylic acid decreases the size of platelets. The Finnish Medical Society guideline on thrombocytopenia states: “Many drugs cause thrombocytopenia relatively frequently… Non-steroidal anti-inflammatory drugs, especially acetylsalicylic acid frequently impair platelet function and bring about a bleeding tendency. This tendency is disproportionately strong among thrombocytopenic patients. Aspirin has an effect on the endothelium. This detrimental effect on malaria is known since 40 years and it is unacceptable that WHO has not highlighted this.51 -53
Aspirin-treated mice are more susceptible to death from Plasmodium chabaudi infection than placebo-treated animals.54 Fever is accompanied by glycogen destruction. This was already discovered more than 100 years ago. Glycogen disappears from the liver during tetanus, diphteria and pneumonia. A natural way of our body to fight parasites and diseases. It makes thus complete nonsense to fight fever with aspirin in the early stages of a malaria infection.55 Aspirin inhibits cyclooxygenase and affects the TNF-α and prostaglandin E2 produced by the malaria parasite. Let’s not forget that only 1% of those infected by malaria die of the disease because the humane immune system is very efficient. Any interference by xenobiotics may have dramatic consequences.56 -58
Acidosis and renal failure are an important cause of death in severe malaria. The intake of aspirin before hospitalization plays a role in the development of acidosis and may exacerbate Plasmodium falciparum infection. Paracetamol vs placebo in treatment of non-severe malaria was studied in Guinea Bissau. It showed that time to parasite clearance is significantly longer in children treated with paracetamol and recrudescence was higher.59 Paracetamol by itself may lead to thrombocytopenia.60 ,61
Artemisia plants
Artemisia plant extracts increase and stabilizes the platelet count in malaria infections and increase survival (G Bamunuarachchi op.cit.). But this is not the case for artesunate monotherapy where platelets count, hemoglobulin, erythrocytes decrease.62 But the action is complex. Some of the constituents of Artemisia have opposing roles. Platelet aggregation is induced by arachidonic acid; pentacyclic triterpenes inhibit aggregation.63
In a trial in Brazil, at the Campinas university, the Artemisia annua tea-taking patients showed shorter bleeding times than those in Coartem group. The bleeding time evaluates the efficiency of the platelets. The patients in the Coartem group presented a mean platelet count much lower than the tea group. Artemisia annua is also a potent inhibitor of TNF-α and PGE2, and may thus reduce the inflammation leading to severe malaria. Probably one of the major reasons why it is important to administer Artemisia annua decoctions during at least seven days to modulate and temper inflammation at the end stage of the malaria infection when most of the platelet population has been deleted.64 In vivo studies in Algeria show that Artemisia extracts have strong anti-inflammatory effects. Similar immunomodulatory effects of Artemisia amygdalina were found by a research team in India. This may be important in the trophozoite stage of Plasmodium falciparum infection where inflammation and fever may lead to severe malaria.65 -67
Artemisia plants are rich in proanthocyanidins, lowering proinflammatory cytokins and TNF-α.68 Plasmodium falciparum extensively remodels its host red blood cell. The zeta potential is an electrochemical property of cell surfaces that is determined by the net electrical charge of molecules exposed at the surface of cell membranes. The RBC membrane is negatively charged and is surrounded by a fixed layer of cations. Using an electrophoretic mobility assay, it was found that the main zeta potential was significantly lower in in RBCs infected with Plasmodium falciparum.69 At high doses paracetamol can be lethal, but this effect can be reversed by Artemisia absinthium extracts. In a trial on mice acetaminophen produced 100% mortality at the dose of 1 g/kg while pretreatment of animals with plant extract (500mg/kg) reduced the death rate to 20%. Pretreatment of rats with plant extract also prevented the acetaminophen induced rise in serum transaminases (GOT and GPT).70 Artemisia plants are rich in saponins. They are considered useful in the prophylaxis and treatment of several diseases and are used as adjuvants in malaria vaccines. Saponin from medicinal herbs also activates calcium-dependent potassium channels. This may induce cell scrambling and eryptosis. The concentrations required for both eryptosis and hemolysis (15μg/ml) are easily achieved in vivo. The stimulation of eryptosis is followed by removal of defective erythrocytes prior to hemolysis. By preceding hemolysis, eryptosis protects against the effects of hemolysis.71 ,72 Artemisia annua is also very rich in potassium (Figure 4).73 There is a strong correlation between thrombocytopenia, thrombocythemia, hypokalemia and hyperkalemia.74
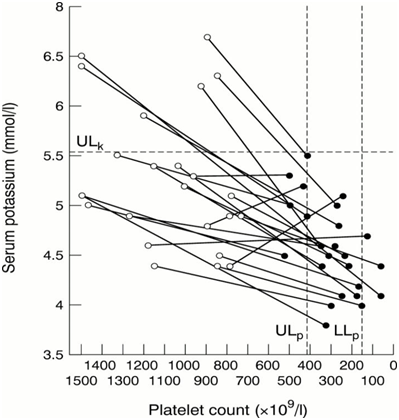
Figure 4 Investigation of possible abnormalities of serum potassium and platelet count in patients with essential thrombocythaemia and significant thrombocytosis.75
But the question remains open: does high potassium content in plasma increase the platelet number or is the low potassium content a consequence of a low platelet count. In our humble opinion the first hypothesis merits further studies. The only confirmation we found for this hypothesis in the scientific literature is that Carica papaya, a known antimalarial plant, very rich in potassium, increases the platelet count; in a very significant manner in a in vivo trial in rats, almost doubling it after 7 days administration of an aqueous extract.76
None.
The author declares that there is no conflict of interest.

©2018 Lutgen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.