eISSN: 2379-6367


Opinion Volume 6 Issue 4
IFBV-BELHERB, Luxembourg
Correspondence: Pierre Lutgen, IFBV-BELHERB, BP 98 L-6905, Niederanven, Luxembourg
Received: June 06, 2018 | Published: July 6, 2018
Citation: Lutgen P. New approaches in malaria prophylaxis: endophytic fungi, asparaginase, potassium and papaya. Pharm Pharmacol Int J. 2018;6(4):275-277. DOI: 10.15406/ppij.2018.06.00186
Malaria infection is initiated when a mosquito injects Plasmodium sporozoites into a mammalian host. Sporozoites exhibit gliding motility both in vitro and in vivo. This motility is associated with the secretion of at least two proteins, circumsporozoite protein (CSP) and thrombospondin-related anonymous protein (TRAP). Several molecules, as for example albumin promote this motility by mechanisms which are not well understood. The use of skin care products containing albumin is thus questionable. Most of these ingredients are derived from soybean.1,2 But there are other molecules which inhibit the motility of sporozoites.
Endophytic fungi or endophytes exist widely inside the healthy tissues of living plants, and are important components of plant micro-ecosystems. Over the long period of evolution, some co-existing endophytes and their host plants have established a special relationship with one and another, which can significantly influence the formation of metabolic products in plants, then affect quality and quantity of crude drugs derived from medicinal plants.3 Liver-stage Plasmodium parasites exhibit one of the fastest nuclear replication rates known among eukaryotic organisms leading to the formation of several thousand progeny. This extensive and rapid growth necessitates the acquisition of many nutrients.4 The proteins of Plasmodium, the malaria parasite, are strikingly rich in asparagine. Plasmodium depends primarily on host haemoglobin degradation for amino acids and has a rudimentary pathway for amino acid biosynthesis, but retains a gene encoding asparagine synthetase. The deletion of asparagines in Plasmodium berghei delays the liver-stage development and leads to a substantial reduction in the formation of ookinetes, oocysts and sporozoites in mosquitoes. Conversion of asparagine into aspartic acid and depletion of blood asparagine levels infected mice with the enzyme asparaginase completely prevents the development of liver stages, ex-flagellation of male gametocytes and the subsequent formation of sexual stages. In vivo supplementation of asparagine in mice restores the ex-flagellation. Thus, the parasite life cycle has an absolute requirement for asparagine, which could be targeted to prevent malaria transmission and liver infections.5,6 Asparagine is an amino acid present in vegetables like asparagus, peas or beans. It is absent in Artemisia annua.7 It is also found in many honeys.8
Asparaginase is an enzyme that is used in medicine and in food manufacturing. As a medication it is used to treat acute lymphoblastic leukemia, acute myeloid leukemia, and non-Hodgkin's lymphoma. The cancer cells thrive on large quantities of asparagine. Asparaginase, however, catalyzes the conversion of L-asparagine to aspartic acid and ammonia. This deprives the leukemic cell of circulating asparagine, which leads to cell death. It will also deprive Plasmodium of the asparagine it badly needs. Additionally, the production of ammonia may present a huge toxicity challenge to a parasite that lack ammonia-detoxifying machinery.9 E. coli strains are the main source of medical asparaginase. It is generally carried out by submerged fermentation, and this may explain why it is present in wines and beers. It is produced at industrial scale by the use of agro-wastes, like peels of onions, of oranges, of garlic. But it is also present in several medicinal plants, Ocimum tenuiflorum, Carica papaya, Azadirachta indica.10,11 Asparaginase was approved for medical use in the United States in 1978. It is an authorized food additive.12 Asparaginase was first detected in the developing seeds of Lupinus albus.13,14
Studies from Thailand and India show that many medicinal plants are rich in asparaginase, and that it is even the predominant enzyme compared to others present like amylase, cellulose, pectinase.15‒18 Artemisia plants are rich in fungal endophytes with a great variety in species.19‒22 Different fungal communities colonize stems and leaves. This may explain why it is claimed that it is important to keep twigs and stems in dried Artemisia annua for tea infusions. Artemisia plants are more than other plants very rich in potassium and don’t contain any sodium (EA Brisibe, op.cit.). There may exist a synergetic effect between potassium and asparaginase in Artemisia plants. A more complete study in Pakistan, comparing 10 medicinal plants finds that potassium content in Artemisia annua is the highest.23‒25 This synergy between potassium and asparaginase was confirmed by a seminal work in Brazil. The enzyme is dependent upon the presence of K⁺ for activity. Maximum activity was obtained at K⁺ concentrations above 20millimolars. Potassium also exerts an import role in the stabilization of asparaginase at elevated temperatures. This stabilizing effect is separate from its activating effect. Under conditions where the enzyme shows a reasonable degree of stability in the absence of K⁺, i.e., at 20°C the enzyme is still inactive unless potassium is present (see Figure 1).26,27
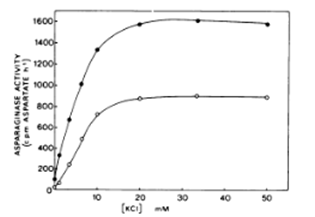
Figure 1 Effect of increasing concentration of KCI on asparaginase activity isolated from test and cotyledon of immature P. sativum seeds.
Papaya has a solid reputation as antimalarial plant not only in South America but also in Africa. As stated in Wikipedia:” In some parts of the world, papaya leaves are made into tea as a treatment for malaria, but the mechanism is not understood”. Most studies deal with leave extracts, but seed extracts have also been demonstrated to be very active in vivo with efficacies similar to that of chloroquine.28‒31 Papaya leaves are a rich source of L-asparaginase when compared to other plants. This may be one of the reasons of its prophylactic and antimalarial properties.32 In Papaya carica like in Artemisia annua, potassium is the mineral present at the highest concentration.33 Cocoa (Theobroma cacao) also contains asparaginase. It was known by the Maya as diet-mediated antimalarial prophylaxis. Based on this anecdotal information prophylactic trials have been started in Ghana by the Ghana Cocoa Board. People are encouraged to daily drink a beverage made by mixing boiling hot water and natural cocoa powder.34,35 This has been confirmed by in vivo trials in mice. Cocoa powder was equivalent to chloroquine and has also prophylactic properties.36 Possible effects of asparaginase on gametocyte motility.
Transmission of Plasmodium parasites to the mosquito requires the formation and development of gametocytes. Studies in infected humans have shown that only the most mature forms of Plasmodium falciparum gametocytes are present in circulation, whereas immature forms accumulate in the hematopoietic environment of the bone marrow. Only mature gametocytes reappear in the peripheral circulation. A recent paper shows that these mature gametocytes show high deformability and the authors suggest that gametocyte mobility is essential for transmission of Plasmodium to the mosquito. Speeds of 5 to 10μm/s were observed. It is comparable with motile “zoite” forms of the parasite such as sporozoites. The deformability and motility of mature gametocytes was blocked by sildenafil citrate.37 Regulated K⁺ transport is of vital importance for the survival of most cells. Two K⁺ channel-encoding genes have been found in Plasmodium falciparum genome.38 Sulfadoxine-pyrimethamine impairs Plasmodium falciparum gametocyte infectivity. It is possible that Artemisia plants also contain molecules which block the mature gametocytes. A study from South Africa shows that compared with eight other medicinal plants Artemisia afra ranks at the top for the inhibition of gametocyte viability.39,40
None.
The author declares that there is no Conflict of interest.

©2018 Lutgen. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.