Open Access Journal of
eISSN: 2576-4578


Research Article Volume 1 Issue 3
1Institute of the Bioorganic Chemistry, Republic of Uzbekistan
2Xinjiang Technical Institute of Physics and Chemistry, China
3Institute of the Chemistry of Plant Substances, Republic of Uzbekistan
Correspondence: Shunkor S Khushmatov, Institute of the Bioorganic Chemistry, Academy of Sciences, Republic of Uzbekistan
Received: September 25, 2017 | Published: November 3, 2017
Citation: Khushmatov SS, Usmanov PB, Inogamov UK, et al. Possible involvement of no–synthase in inotropic effect of 4’,5–dihydroxy–7–methoxyflavon in rat cardiac muscle. Open Access J Trans Med Res. 2017;1(3):69-74 DOI: 10.15406/oajtmr.2017.01.00014
The aim of the present study was to determine the inotropic effect of 4',5–dihydroxy–7–methoxyflavon in the rat myocardium. The present study demonstrates that 4',5–dihydroxy–7–methoxyflavon (1–5µmol.L–1) showed a positive inotropic effect on rat papillary muscles that can be explained with the increase of [cAMP]in and may depend on increase of [Са2+]in. Also, another possible mechanism of the positive inotropic effect 4',5–dihydroxy–7–methoxyflavon (5µmol.L–1) on the rat papillary muscles can be inhibited Na+/K+–ATPase via activation of reverse–function of the Na+/Ca2+–exchange and, consequently, increase [Са2+]in. And 4',5–dihydroxy–7–methoxyflavon (10–50µmol.L–1) may induce a negative inotropic effect in rat cardiac muscles and this effect at least partly be mediated by NO/cGC/cGMP/PKG pathway.
Keywords: biphasic inotropic effect, papillary muscles, nitric oxide, no/cgc/cgmp/pkg, 4',5–dihydroxy–7–methoxyflavon
RyR, ryanodine receptors; SERCA, sarcoplasmic reticulum; PLN, phospholamban; ATP, adenosintriphosphate; AC, adenylate cyclase; cAMP, cyclic adenosine 3',5'–monophosphate; PKA, proteine kinase a; cGMP, cyclic guanosine monophosphate; NO, nitric oxide
Treatment of cardiovascular diseases includes a wide range of pharmacological drugs, and plant remedies are suitable alternatives to synthetic drugs due to their availability, non–proarrhythmic characteristics, and minimal side effects.1 Medicinal plants distributed in the Central Asia and China provides abundant and precious medicinal resources for the Traditional Medicine in treating diseases.2 Flavonoids are unique secondary metabolites are synthesized in almost all plant cells, exhibiting high biological activity and due to their properties increasingly finding wide practical application in pharmacology and medicine. A wide range of biological activities and low toxicity puts them in a row promising compounds in this respect. Therefore, in pharmaceutical industry researches find cure against many various diseases, the direction of tendency is observed towards the growth of interest to search and create medical products based on bioflavonoids.3–8 Also, nowadays, in chemical researches have extracted and identified many bioflavonoid from plants.7,9–12 However, their mechanisms of cardio pharmacological impact have not been studied yet. Therefore, the aim of the present study was to involvement of NO–synthase in inotropic effect of 4',5–dihydroxy–7–methoxyflavon in rat papillary muscle.
Animals and ethics statement
This study was carried out in the Laboratory of Pharmacology of Institute of Bioorganic Chemistry of Academy Sciences of the Republic of Uzbekistan on physically fit, adult, albino rats in both sexes (female and male) obtained from the vivarium in the Laboratory of Pharmacology. Animals had been fed with standard food and water in the vivarium. In all experiments albino rats weighing 150–200g were used (n=11). During the experiments, while working with experimental animals, International principles of the Helsinki Declaration and the rules of human attitudes towards animals were completely followed.
Solvents and chemicals
All reagents, which were used in experiments, were of analytic–grade (NaCl, KCl, CaCl2, MgSO4, KH2PO4, glucose, NaHCO3), (±)–propranolol hydrochloride, dimethylsulfoxide (DMSO) was obtained from Sigma–Chemical (St. Louis, Missouri, USA). Ca2+L–channels were inhibited by 0.01–1µmol.L–1 nifedipine hydrochloride (Sigma Aldrich; Germany).13 Also, β–AR was inhibited by 10µmol.L–1 (±)–propranolol hydrochloride. 4',5–Dihydroxy–7–methoxyflavon was isolated from plants Dracocephalum komarovii Lipsky (Lamiaceae), collected from the Tashkent region of Uzbekistan and presented by PhD. Students of CAS_TWAS president fellowship Chinese Academy of Sciences (Xinjiang Technical Institute of Physics and Chemistry) Zokir Toshmatov (Figure 1).
Dracocephalum komarovii Lipsky (Labiatae) is endemic species, groving only in territory of Republic Uzbekistan. Dracocephalum komarovii Lipsky– a perennial semishrub that grows in the vicinity of ~2300–3600 m above sea level in the western Tien Shan mountain system. This is called «buzbosh» in Uzbekistan, and the local people use the aerial parts in a tea to treat various diseases such as inflammatory diseases and hypertension.
4',5–dihydroxy–7–methoxyflavon were dissolved in dimethyl sulfoxide (DMSO<0.1% in the bath). L–NAME (Nω–nitro–L–arginine methyl ester, Sigma–Aldrich, Germany), (±)–propranolol hydrochloride and nifedipine hydrochloride were all dissolved in distilled water. In the experiments, modified the physiological Krebs–Henseleit solution containing (in mM): 118 – NaCl; 4.7 – KCl; 2.5 – CaCl2; 1.2 – MgSO4; 1.1 – KH2PO4; 5.5 – glucose and 25 – NaHCO3; pH 7.4 were used. This Krebs–Henseleit solution which was continuously bubbled with 95% O2 and 5% CO2 and kept at a temperature of +36±0.5°C by means of water heating system controlled by temperature controller U1 (Russia), and flowed in and out of the organ bath at a rate of 3–5ml/min with the peristaltic pump LKB Bromma (Sweden).
Preparation of tissue and measurement of contractility and setup of the equipment
In experiments the papillary muscles preparations, isolated from the right atrium of adult albino rats’ hearts. Rats were deeply anaesthetized with diethyl prior to paralyzing by using cervical dislocation method. The papillary muscles were ~0.4–1.3mm in diameter and 2.5–3.8mm in length. Isometric tension forcesG20, World Precision Instruments, Inc. 175 Sarasota Center Boulevard, Florida 34240–9258, USA), designe were recorded using a force transducer (SI–Kd for the in vitro study in standard pharmacological experiments for measuring contraction force response of isolated muscle preparations. The organ chamber (20ml) was part of the experimental setup, as shown in Figure 2. For further details of setup of the recording system is given in the text.
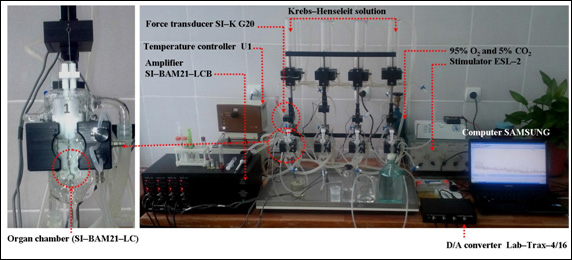
Figure 2 Schematic drawing of the experimental setup of the recording system for contractile activity of the isolated papillary muscle.
The Isometric force transducer SI–KG20 is connected to a transducer amplifier (SI–BAM21–LCB, WPI, Inc. 175 Sarasota Center Boulevard, Florida 34240–9258, USA). The papillary muscle was lifted with electric impuls that was higher than threthold (~20%), rectangular, electrical pulses of frequency 0.5Hz; 5–10msec and 5V amplitude, delivered via a pair of platinum electrodes placed in the muscle–mounting organ chamber by using stimulator ESL–2 (Russia).
Thus, wires of a pair of platinum electrodes were placed as parallel to the organ; the physiological solution of Krebs–Henseleit provides shortening the electrical contact distance between the electrodes and the preparate of the papillary muscles. In this experiment, 10mN (1g~9.8mN) was accepted for a resting tension of the preparation papillary muscles.
After a 45–60minutes equilibration period, the length that provides development of the maximal isometric contractile force (Lmax, the maximal length) length of the papillary muscle was found, and all experiments were carried out in these condition. After the equilibration period in the organ chamber, papillary muscles were stimulated by an initial electrical pulse of frequency 0.5Hz, amplitude 5V, and 5msec pulses. The signals obtained were given from the transducer SI–KG20 to amplifier and sent to a computer with analogue–digital converter Lab–Trax–4/16 (WPI, USA) software (iWorx LabScribe2; iWorx Systems, Inc., USA).
Data analysis
Papillary muscle contractions were plotted as a percentage of the force before the drug application in each muscle. Data were analyzed by Origin Lab Origin Pro v. 8.5 SR1 (EULA, Northampton, MA 01060–4401, USA). Pooled data are given as means ±S.E.M. of observations (n). Concentration–response curves were fitted to the logistic equation:
where Emax – is the maximal effect, k – is a factor which represents the slope of the curve, and pD2 – is the drug concentration exhibiting 50% of the Emax expressed as negative log molar. Values are expressed as mean±S.E.M. Statistical differences of the data were calculated by ANOVA and the paired or unpaired Student’s t–test where appropriate. The values were considered significantly different when p<0.01 and p<0.05.
The positive inotropic effects of 4',5–dihydroxy–7–methoxyflavon on rat papillary muscle
In the experiments, the 4',5–dihydroxy–7–methoxyflavon (1–50µmol.L–1) showed dose–dependent biphasic inotropic effects in rat papillary muscle contractility.
The condition where the maximal effected concentration (5µmol.L–1) of 4',5–dihydroxy–7–methoxyflavon, the isometric developed force of papillary muscle preparation was increased from 2.6±0.1 mN (the control basal value) to 3.8±0.1mN or 46.2±4.78% in comparison with the control group (p<0.05; n=3–5).
Studies have shown that in incubation to inhibited potential–dependent Са2+–channel – nifedipine hydrochloride (0.01µmol.L–1) the positive effect of 4',5–dihydroxy–7–methoxyflavon (5µmol.L–1) decreases to 31.5±4.7% of control values. The results of experiment demonstrate that the positive inotropic effect of 4',5–dihydroxy–7–methoxyflavon on papillary muscle is not completely connected with modulation of potential–dependent Са2+L–channel cardiomyocytes. In addition, the positive inotropic effect of 4',5–dihydroxy–7–methoxyflavon (5µmol.L–1) was almost completely disappeared in the presence of inhibitor of potential–dependent Са2+L–channel–nifedipine hydrochloride (0.01µmol.L–1) and inhibitor β–adrenoreceptor–(±)–propranolol hydrochloride (10µmol.L–1) under incubation conditions.
Other investigations showed that flavonoids demonstrate a positive inotropic effect, through an increase in cAMP, which increases [Ca2+]in. It is known that, increasing concentration of [cAMP]in activates protein kinase A (PKA), which phosphorylates the L–type Ca2+–channel, troponin I, and causes an increase of [cAMP]in and subsequently phosphorylation of contraction–controlling proteins, including Ca2+L–channels occured, and the amplitude force of contraction papillary muscles increased as well. Phosphorylation of these Ca2+L–channels promotes Ca2+ influx that triggers the release of Ca2+ from the RyRs of SR and [Ca2+]in transient finally activates the contraction system of myocardium.
The present study demonstrates that 4',5–dihydroxy–3,6,7–trimethoxyflavon has showed a positive inotropic effect on rat papillary muscles that can be explained with the increase of [cAMP]in and may depend on increase of [Са2+]in.
Another possible source of [Са2+]in could be found in activation of reverse–function of the Na+/Ca2+–exchange.14 Also, cardiac glycosides (ouabain, digoxin et al.) exert a positive inotropic effect on papillary muscle through a mechanism the inhibition of the Na+/K+–ATPase (sodium pump). This leads to an increased [Na+]in which in turn is thought to influence the Na+/Ca2+–exchange in such a way as to increase the [Ca2+]in and hence the force of contraction papillary muscle.15–21 And, inhibition of Na+/K+–ATPase is involved in probably to a large extent in therapeutic efficacy of cardiac glycosides.20,22,23
The most frequently cardiac glycoside used in experimental research and in clinical conditions today is digoxin.24–28 Digoxin, a cardiac glycoside and digitalis alkaloid obtained from the leaves of plants of the plant Digitalis lanata, and used clinically in the treatment of heart failure and arrhythmias by inhibiting the Na+/K+–ATPase by binding to the extracellular domain of the α–subunit of Na+/K+–ATPase on cardiomyocytes. And, reported29 that digoxin at 0.4micromol.L–1 demonstrated a near maximal positive inotropic effect (EC50=~0.2µmol.L–1).
Given that to examine this possibility, the Na+/K+–ATPase was modified by incubating papillary muscle with 1micromol.L–1 digoxin. Thus, papillary muscle from rat displayed an increase of force to 87.4±6.7% of control values after digoxin (1micromol.L–1; 0.5Hz) (n=3–4; p<0.05). A combination of 1µmol.L–1 digoxin and 4',5–dihydroxy–7–methoxyflavon (5µmol.L–1) resulted in a potentiation of the developed contractile tension of the rat papillary muscles from to 15.8±4.9% of control values.
Also, reported30 that flavonoids demonstrated a positive inotropic effect, through an inhibited Na+/K+–ATPase in vitro.
These results indicate that another possible mechanism of the positive inotropic effect 4',5–dihydroxy–7–methoxyflavon (5µmol.L–1) on the rat papillary muscles can be inhibited Na+/K+–ATPase via activation of reverse–function of the Na+/Ca2+–exchange via increase [Са2+]in.
The negative inotropic effects of 4',5–dihydroxy–7–methoxyflavon on rat papillary muscle
In the experiments, the 4',5–dihydroxy–7–methoxyflavon (15–50µmol.L–1) induced a concentration–dependent decrease of the contractile force of the rat papillary muscle (p<0.05). The condition where the maximal effected concentration (50µmol.L–1) of 4',5–dihydroxy–7–methoxyflavon, the isometric developed force of papillary muscle preparation was decreased from 54.8±6.2% in comparison with the control group (p<0.05; n=3–4) (Figure 3).
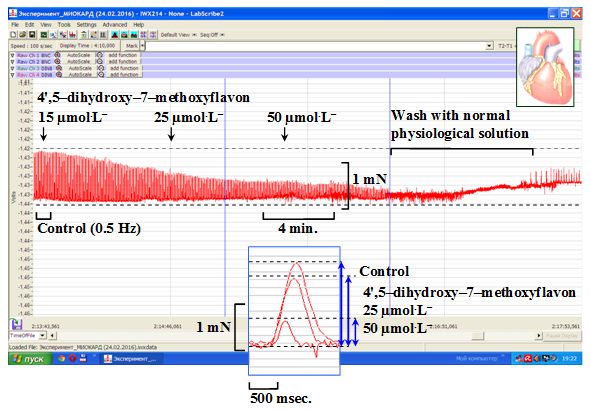
Figure 3 Original recordings of negative inotropic effect of 4',5–dihydroxy–7–methoxyflavon (15–50 µmol.L–1) on a rat papillary muscle.
Stimulation: 0.5Hz with pulses of amplitude 5V, and 5msec (+36±0.5°C).
In these conditions, the EC50 value (the values of concentration for 50% of the maximal effect) 4',5–dihydroxy–7–methoxyflavon was 38.9µmol.L–1 or pD2 (–logEC50)=4.415 (Figure 4).
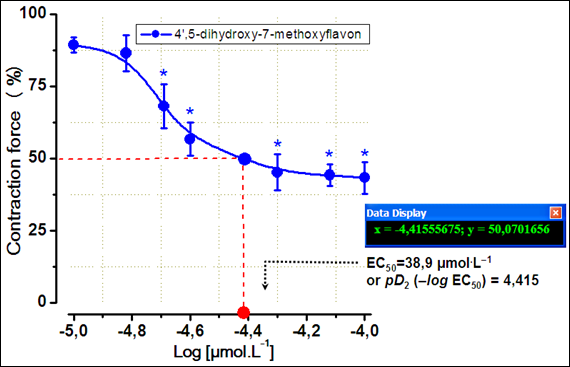
Figure 4 Concentration–response curves for the negative inotropic effects of 4',5–dihydroxy–7–methoxyflavon.
Results are given as means±S.E.M, n=3–4. Stimulation: 0.5Hz, 5V, 5msec, +36±0.5°C, resting tension=10mN.
In the experiments, the NO donor sodium nitroprusside (SNP) (100µmol.L–1) showed a negative inotropic effect (at 0.5Hz; p<0.05). Treatment with sodium nitroprusside (SNP) (100µmol.L–1) decreased maximal force by 56.4±4.6% compared to control conditions. These results are consistent with studies other authors.31 This effect is nearly eliminated by the inhibition of NO–synthases through L–NAME (100µmol.L–1).
Pretreatment with L–NAME (100µmol.L–1), an inhibitor of NO–synthase, attenuated the negative inotropic effect of 4',5–dihydroxy–7–methoxyflavon in the isolated rat papillary muscles (p<0.05) (Figure 5).
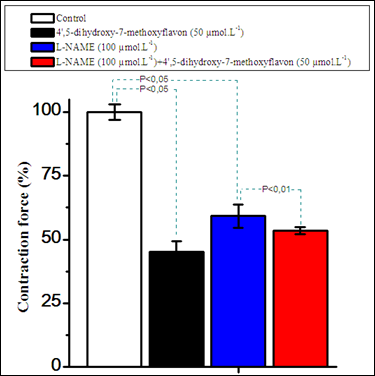
Figure 5 Comparison of the inotropic effects of 4',5–dihydroxy–7–methoxyflavon and L–NAME (100 µmol.L–1) on the contraction force of extracted rat papillary muscle.
Stimulation: 0.5Hz, 5V, 5msec, +36±0.5°C, resting tension=10mN. Data shown for L–NAME (100µmol.L–1) and 4',5–dihydroxy–7–methoxyflavon (50µmol.L–1) were presented as means±of 3–4 experiments. p<0.01 and p<0.05 indicates value compared to control (n=3–4).
And, the negative inotropic effect of 4',5–dihydroxy–7–methoxyflavon (10–50µmol.L–1) in isometrically contracting papillary muscles seems to be mediated by NO/cGMP/PKG–cycle.
Cardio–pharmacological experimental studies in conditions in vitro/in vivo and traditional medical literature point to the cardiovascular effects of bioflavonoids in many instances. All results indicated the potential inotropic and anti–arrhythmic effects of flavonoids in treating cardiovascular diseases. Thus, reported, that the bioflavonoids have a positive inotropic effect, through an increase [cAMP]in, with inhibited of phosphodiesterase (3'–5'–cAMP–phosphodiesterase) enzyme, also with activation of β–AR, consequently, increase [Са2+]in.32–37
And, some bioflavonoids have an inhibitory effect on the Na+/K+–ATPase Na+/K+–ATPase via activation of reverse–function of the Na+/Ca2+–exchange and, which is increase [Са2+]in .37,38,39
In conclusion, the present study demonstrates that 4',5–dihydroxy–7–methoxyflavon has showed a positive inotropic effect on rat papillary muscles that can be explained with the increase of [cAMP]in and may depend on increase of [Са2+]in. Also, another possible mechanism of the positive inotropic effect 4',5–dihydroxy–7–methoxyflavon (5µmol.L–1) on the rat papillary muscles can be inhibited Na+/K+–ATPase via activation of reverse–function of the Na+/Ca2+–exchange which increase [Са2+]in. And 4',5–dihydroxy–7–methoxyflavon (10–50µmol.L–1) may induce a negative inotropic effect in rat cardiac muscles and this effect at least partly be mediated by NO/cGC/cGMP/PKG pathway (Figure 6).
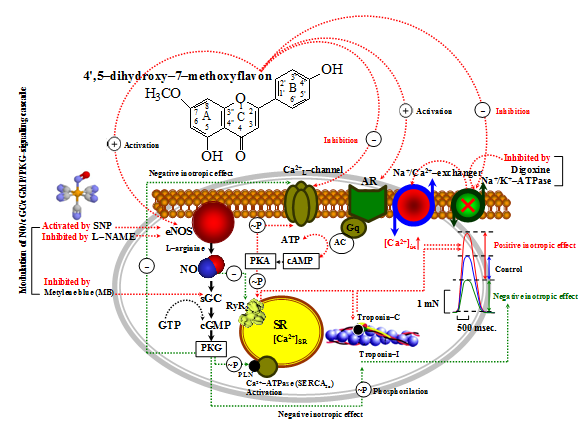
Figure 6 Hypothetical mechanisms of inotropic effect of 4',5–dihydroxy–7–methoxyflavon on the rat myocardium.
RyR – Ryanodine Receptors or Sarcoplasmic reticulum Ca2+–channels; SERCA2a – The sarcoplasmic reticulum Ca2+–ATPase; PLN – Phospholamban; ATP – Adenosintriphosphat; Gs – Guanine nucleotide–binding proteins; AC – Adenylate cyclase; cAMP – Cyclic Adenosine 3',5'–monophosphate; PKA – Proteine kinase A; cGMP – Cyclic guanosine monophosphate; PKG –cGMP–dependent protein kinase. Thus, depolarization of sarcolemmal membrane by AP leads to opening of Ca2+L–channels, which allows entry of Ca2+ into cardiomyocytes. And, through Ca2+–induced activation RyR2, leads to increase in [Ca2+]in, leading to contraction of myofilaments. During relaxation, [Ca2+]in is accumulated into SR by SERCA2a and extruded by Na+/Ca2+–exchanger. Agonists β–AR through G–proteins increase [cAMP]in, which results in activation of PKA, which leads to phosphorylation of Ca2+L–channels, allowing increase [Ca2+]in, phosphorylation of PLN, increasing SERCA2a activity, induce lusitropic effect.
Also, phosphorylation RyR2, induce activation release of Ca2+ from SR, and allowing increase [Ca2+]in. Digoxin inhibits Na+/K+–ATPase, which increases [Na+]in, and this results in increase in [Ca2+]in via Na+/Ca2+–exchanger, which leads to activation SERCA2a and increase in Ca2+ release via RyR2.40 NO (nitric oxide) is an important messenger that regulates physiological functions throughout the cardiovascular system,41 and NO which increase the [cGMP]in level, decreased the contraction force in isolated cardiac muscle.31 The negative inotropic action mediated by cGC activation results from cGMP–dependent protein kinase (PKG) activation and inhibition of cAMP–phosphodiesterase (PDE III), with concomitant Ca2+ myofilament responsiveness reduction by troponin–I. Also, the decreased twitch tension can be due to a PKG–dependent reduction in myofilament responsiveness to Ca2+ in the modulation of NO.42,43 These data may serve as a basis for further detailed pharmacological mechanism of action of this compound.
This study was supported by the Project No. FA–F–6–004 (Uzbek Academy of Sciences), and supported by the International Cooperation and Exchanges of the National Natural Science Foundation of China (Grant No. 31110103908) and the Central Asian Centre of Drug Discovery & Development of Chinese Academy of Sciences, also for CAS TWAS scholarships.
The author declares no conflict of interest.

©2017 Khushmatov, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.