MOJ
eISSN: 2574-9773


Review Article Volume 1 Issue 2
University of Graz, Austria
Correspondence: Martin Koller, University of Graz, Office of Research Management and Service, c/o Institute of Chemistry, Heinrichstrasse 28/III, 8010 Graz, Austria, Tel -6116
Received: February 13, 2017 | Published: June 1, 2017
Citation: Koller M. Production of Poly Hydroxyalkanoate (PHA) biopolyesters by extremophiles? MOJ Poly Sci. 2017;1(2):69-85. DOI: 10.15406/mojps.2017.01.00011
The article reviews the current state of knowledge of the production of polyhydroxyalkanoate (PHA) biopolyesters under extreme environmental conditions.
Although PHA production by extremophiles is not realized yet at industrial scale, significant PHA accumulation under high salinity or extreme pH- or temperature conditions was reported for diverse representatives of the microbial domains of both Archaea and Bacteria. Several mechanisms were proposed to explain the mechanistic role of PHA and their monomers as microbial cell- and enzyme protective chaperons and the factors boosting PHA biosynthesis under environmental stress conditions. The potential of selected extremophile strains, isolated from extreme environments like glaciers, hot springs, saline brines, or from habitats highly polluted with heavy metals or solvents, for efficient future PHA production on an industrially relevant scale is assessed based on the basic data available in the scientific literature. The article reveals that, beside the needed optimization of other cost-decisive factors like inexpensive raw materials or efficient downstream processing, the application of extremophile production strains can drastically safe energy costs, are easily accessible towards long-term cultivation in chemostat processes, and therefore might pave the way towards cost-efficient PHA production, even combined with safe disposal of industrial waste streams. However, further challenges have still to be overcome in terms of strain improvement and process engineering aspects.
Keywords: archaea, bacteria, biopolymers, extremophiles, halophiles, polyhydroxyalkanoate, psychrophiles, thermophiles
µmax, maximum specific growth rate; 16S rRNA, 16 svedberg rribosomal RNA sequencing; 3HB, 3-hydroxy butyrate; 3HD, 3-hydroxy decanoate; 3HDD, 3hydroxy docecanoate; 3HHx: 3-HydroxyHexanoate; 3HN: 3-HydroxyNonanoate; 3HO: 3-HydroxyOctanoate; 3HV, 3hydroxy valerate; 3UD, 3-hydroxy undecanoate; 4HB, 4-hydroxy butyrate; AC, acetyl-coa synthetase; BOD, biochemical oxygen demand; b-PHA, blocky structured polyhydroxyalkanoate; CBF, cyclic batch fermentation; CDM, cell dry mass; CFBF, cyclic fed-batch fermentation; COD, chemical oxygen demand; CT, coa transferase; DSC, differential scanning calorimetry; ECS, extruded corn starch; EPS, extracellular polysaccharides; ERB, extruded rice bran; FT-IR, fourier transform infrared spectroscopy; GBL, γ-butyrolactone; GC-MS, gas chromatography coupled to mass spectroscopy; Hm, melting enthalpyk; kDa, 103 dalton; LPS, lipopolysaccharides; MCL, medium chain length; MW, weight average molecular mass; NADH, nicotinamide adenine dinucleotide; NADPH, nicotinamide adenine dinucleotide phosphate; NMR, nuclear magnetic resonance; PCR, polymerase chain reaction; PHA, poly hydroxyalkanoate; PHB, poly (3-hydroxy butyrate); PHBHV, poly(3-hydroxybutyrate-co-3-hydroxy valerate); PHV, poly (3-Hydroxy valerate); Pi, polydispersity; qp, specific production rate; scl, short chain length; SDS, sodium dodecyl sulfate; SEM, scanning electron microscopy; SSCAs, star-shaped cell aggregates; STEM, scanning transmission electron microscopy; TCA, citric acid cycle; TDS, total dissolved solids; TEM, transmission electron microscopy; Td, onset of polymer decomposition temperature; Tg, glass transition temperature; Tm, melting temperature; Xc, degree of crystallinity
Nowadays, we witness innumerable efforts globally to make processes of “White Biotechnology” ever more cost efficient. In this context, we talk about the bioproduction of diverse bulk and niche products based on the conversion of renewable resources by the action of living organisms or by their biocatalytically active components. Bioproduction of several important products, both from the primary or the secondary metabolism of diverse microbial species, e.g., ethanol, acetic acid, citric acid, bacteriocins, lactic acid, bacterial cellulose, xanthan, or fungal antibiotics is considered as state of the art.1 In contrast, the bioproduction of products with plastic-like properties is still awaiting its ultimate success on the market.2 In this context, polyhydroxyalkanoates (PHA) are biologically synthesized polymers of hydroxyalkanoic acids (Figure 1 ; they act as intracellular carbon- and energy storage materials found in a broad number of Archaea and Bacteria, and provide the cells with an advantage for survival under harsh environmental conditions, such as starvation, exposure to radiation, desiccation, toxic solvents, high salinity, heavy metals, or oxidants. Observed microscopically, they are well visible as bright refractive granular inclusions (Figure 2). In dependence on their composition on the monomeric level, PHA´s material characteristics are similar to those of well-known thermoplastics and elastomers of petrochemical origin. As their outstanding and unique feature, PHA are recognized as the only group of plastic-like materials which a completely biological life cycle, evidenced as follows (reviewed by Koller M et al.3):
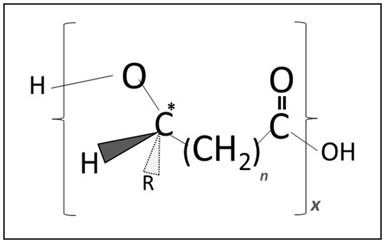
Figure 1 General chemical structure of PHA biopolyesters. R symbolizes the side chain of individual monomeric building blocks (hydroxyalkanoic acids), n accounts for the number of methylene group in the monomer, representing the “backbone” length of the monomer, whereas x represents the degree of polymerization.
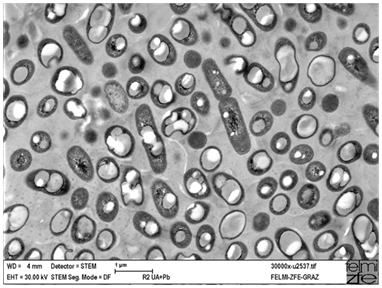
Figure 2 Metalophil production strain Cupriavidus necator DSM 545 with PHA granules (bright intracellular inclusions) imaged by STEM, magnification x 30,000. By curtesy by E. Ingolić, FELMI-ZFE Graz.
The ecological advantages of PHA over their petrochemical competitors which, in most cases, display highly recalcitrant full-carbon-backbone polymers, is evidenced by a number of recent scientific studies, mainly based on modern tools of Life Cycle Assessment, such as the Sustainable Process Index.4–10 Nevertheless, in order to make PHA production competitive also in economic terms, a number of process steps have to be optimized. Efforts in this direction are to an increasing extent devoted to the selection of inexpensive carbon feedstocks, which can replace costly raw materials typically used for biotechnological purposes, such as sugars or edible oils.11 Among such inexpensive feedstocks, a number of [agro] industrial surplus materials was already investigated for PHA production, such as waste streams from dairy and cheese making industry,12,13 residues of the biodiesel and animal processing industry,14–16 or various abundant lignocellulosics,17 e.g., hydrolyzed bagasse and fruit peels,18 straw,19,20 or even spent coffee ground.21 Further, novel processes to recover PHA from microbial biomass are currently in status of development, predominately aiming at the reduction of the input of chemicals and solvents, and at the conservation of the native polymer properties, hence, the PHA´s molecular mass and its quasi-amorphous state.22–24 In addition, new process engineering strategies to increase productivity and to optimize the polyester composition on the monomeric level are currently in status of development; here, one-, two, and multistage continuous process-engineering concepts, optimally matching the kinetic characteristics of PHA biosynthesis (microbial growth phase followed by PHA-accumulation phase, the latter provoked by nutritionally unbalanced conditions together with excess feeding of exogenous carbon source), were designed in the recent years.25,26
Not only during PHA production, but in many biotechnological processes, microbial contamination displays a major risk for running fermentation batches and thus endangers the economic feasibility of new processes in development.27 In this context, the application of extremophile production strains enables the cultivation under drastically reduced or even without any sterility precautions. In the case of the halophile PHA-producer Haloferax mediterannei28 or the thermophile Chelatococcus sp.,29 fermentation batches were operated without sterilizing the bioreactor equipment, and were operated monoseptically for extended time periods. Cultivation processes of thermophilic microorganisms are considered more energy-efficient due to less energy required for cooling. By the high thermal energy generated by the cell´s metabolism, especially during high cell density cultivation, such thermophilic set-ups display “self-heated” systems. Heat input generated by the bioreactor´s stirring system also contributes to the heating of the fermentation process itself. This makes clear that both heating and cooling costs can be reduced and, similar to the above mentioned application of halophiles, sterile conditions may not be essential during a process involving thermophilic microbes. In addition, the use of production strains which thrive well at extreme pH-values, far away from the pH-optimum of potential contaminants, minimizes the risk of microbial contamination; more specifically, the use of alkaliphile production strains avoids the occurrence of fungal infections.30 In addition to their biotechnological significance, alkaliphile organisms attract interest in bioremediation processes, e.g., in neutralizing alkaline waste and removing contaminations of heavy metals.31
The potential of both microbial wild-type strains and genetically modified uni- and multicellular organisms (prokaryotes, yeasts, plants, etc.) for PHA production is exhaustively described in the scientific literature. Microbes are characterized by different optimum conditions for their cultivation. In most cases, biotechnology resorts to such organisms best to be farmed under mesophilic conditions, hence with optima for the pH-value near the neutral range, moderate salinities and temperature between approximately 25 and 37°C, whereas extremophiles thrive best under conditions that would destroy or inactivate most other organism and, in many cases, cannot even survive in normal environments. Extreme environments encompass low [-2 to 20°C] or high [55 to 121°C] temperature, high salinity (2-5 M NaCl), high acidity (pH-value below 4), or high alkalinity (pH-value exceeding 8) (reviewed by Gomes J et al.30). During the last decades, increasing interest is noticed for biotechnological processes to be carried out under extreme conditions. A range of products, such as thermostable “extremozymes”, ectoins, polysaccharides, or pigments can be obtained from extremophile organisms, and be applied for various marketable products.32 The quest for both Gram-positive and Gram-negative microbial species to be used as extremophile cellular PHA-bio polyester factories started in the middle of the 1980 ies by the discovery of the high PHA accumulation potential of the extremely osmophilic Archaeon Hfx. mediterannei, a versatile PHA-,33 pigment-,34 halocin-,35 and polysaccharide,36,37 producer from the haloachaeal group of extremophiles.38 Pigment- (C50 carotenoids) and extracellular polysaccharide (EPS) production provides cultures of these organisms a typical reddish and mucous character, as illustrated in Figure 3. This high versatility of Hfx. Mediaterranei was the starting point for the global quest for other extremophiles with high biopolyesters production capacity. The subsequent paragraphs invite to a journey into significant R&D activities accomplished in this field in the past and the recent years, and are devoted to elucidate mechanisms, biological functions and perspectives for industrial scale implementation of PHA production by extremophiles in a not too distant future.
Halophile PHA producers
General aspects about halophiles: Halophiles are a phylogenetically versatile group of microbes requiring hypersaline environments with NaCl acting as the major salt component. Nature developed different strategies to adapt living organisms to life in highly saline environments. The predominant approach comprises the accumulation of organic compatible osmotic solutes without at the same time requiring special adaptation of intracellular proteins to the high salinity.39 The manifold strategies of halophile microbes to handle highly saline environments, and the fact that halophilicity occurs throughout the tree of life, suggests that adaptation to high salinity is, from the metabolic point of view, a rather trivial task. Changing pigment patterns in microalgae, which, besides illumination conditions, also is determined by the salinity, is regarded a prime example of the adaptive response to changing environmental conditions.40 In the case of halophile PHA producers, Soto and colleagues reveled the role of PHA as chaperons, preventing protein agglomeration under combined stress exerted by salt and temperature, by investigating Pseudomonas sp. CT13, a halotolerant bacterium, and it’s not PHA accumulating mutant. PHA was found to be essential to salt stress resistance, and its productivity positively correlated with salt concentration, evidencing its role as a compatible solute in Pseudomonas sp. CT13.41
The application of halophile microbes in biotechnology opens the door for several options: Firstly, high salinity of cultivation media minimizes the risk of microbial contamination. Secondly, growing halophiles in saline media to high cell densities enables the concentration of salt inside the cells, hence, the reduction of salinity in the cultivation medium. This becomes important in the case of cultivating cells on highly saline waste streams. Thirdly, HCl-catalyzed hydrolysis of inexpensive raw materials to generate feedstocks for PHA production, as demonstrated in the past for bagasse,18 whey,42 straw,19,20 spent coffee ground,21 or “liquefied wood”43 requires neutralization of the cocktail of hydrolysis products. This neutralization, typically accomplished by addition of NaOH, generates considerable amounts of NaCl which contributes to the salinity of the fermentation medium when added as substrate.
Gram-negative halophile PHA producers: The first process for PHA production based on the use of the extremely halophile Archaeon Hfx. mediterannei, an organism originally isolated from ponds of a solar saltern near Santa Pola at the Iberian coast of the Mediterranean sea, already underlined the positive effect of the used unusual culture medium, containing more than 20% NaCl, to prevent contamination by alien microbes.44 The high osmotic pressure of this strain was also used to release PHA granules from the surrounding cell mass via a simple approach: salt concentration below 10% already caused partial cell lysis; exposure to hypotonic media (distilled water) completely disrupts the cells due to their high inner-osmotic pressure, and sets free the storage material (PHA granules). Separation of PHA granules from cell debris can easily be accomplished by profiting of the density difference of these two fractions, thus simplifying decantation and centrifugation. This allows designing extremely simple production facilities. In contrast to other extremophiles known at that time, the organism displays fast growth, relatively high specific PHA productivity and excellent product quality in terms of low degrees of crystallinity (Xc), low melting temperature (Tm), high molecular mass, and narrow molecular mass distribution (low polydispersity Pi).45 In a continuous chemostat cultivation at a dilution rate of 0.121/h, and a temperature of 38°C, a PHA concentration of 6.5g/L was obtained using 20g/L starch as carbon source, which is almost twice the value obtained by using glucose (3.5g/L).45 Astonishingly, the strain produced a high-quality poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBHV) copolyester from simple carbon sources such as glucose or starch; normally, 3-hydroxyvalerate (3HV) synthesis requires the supplementation of structurally related precursor compounds like odd-numbered fatty acids (propionic acid, valeric acid, etc.), which contributes considerably to PHBHV´s production cost. This unique metabolic feature is based on particularities of the strain´s PHA-biosynthesis pathway, which accumulates high intracellular pools of the 3HV-precursor propionyl-CoA.46 In 2006, Don and colleagues fractionated the PHBHV by using a chloroform/acetone mixture and revealed that the polymer consisted of two fractions of different monomeric composition; the predominant fraction (about 93wt.-% of the total PHA) contained about 10.7%(mol/mol) 3HV, whereas the smaller fraction has a higher 3HV content of about 12.3%[mol/mol] and a drastically lower molecular mass (78.2kDa vs. 569.5kDa, respectively), making it soluble even in the classical “PHA-non solvent” acetone. Both fractions displayed low Pi, and similar Tm and glass transition temperature Tg. Via Differential Scanning Calorimetry (DSC), the authors noticed two overlapping melting peaks at heating rates below 20°C/min. The relative intensity of these two melting peaks varied by changing the heating rate, hence, it was assumed that the phenomenon is caused by a melt/recrystallization process.47
Based on these pioneering explorations, Hfx. mediterannei-mediated PHA production has been further optimized by researchers all around the world. Whereas some of these groups focused on the application of inexpensive carbon feedstocks to safe substrate costs, such as whey permeate from dairy industry,12,13,48 rice-based ethanol stillage,49 extruded rice bran,50 enzymatically extruded starch,51 crude glycerol phase,28 olive mill waste water,52 vinasse,53 etc., others clarified the enzymatic and genetic background of PHA-synthesis54–57 and in vitro degradation58 by this organisms. Mathematic models of PHA-production,59 and kinetic studies of PHA and by-product synthesis and degradation60 were reported for Hfx. mediterannei. Only recently, Han and colleagues reported that blocky structured copolyesters (b-PHA), consisting of blocks of PHB and poly (3-hydroxyvalerate) (PHV), linked to randomly distributed PHBHV blocks, can be produced by this organism by optimized co-feeding of glucose and valeric acid. The “blocky” feature and the high 3HV fraction of b-PHA provide this novel copolymer with special material features, encompassing enhanced crystallinity and improved Young’s modulus. Due to the fact that the b-PHA exhibited increased platelet adhesion and accelerated blood clotting compared to random PHBHV, the material was suggested for medical application.61
Due to its high robustness and stability of the culture, Hfx. mediterannei is currently considered the most promising candidate for whey-based PHA production on industrial scale.12 The strain grows excellent on hydrolyzed whey permeate with a maximum specific growth rate (μmax.) of 0.111/h, an outstandingly value for haloarchaea. PHBHV was synthesized at a maximum specific production rate (qP) of 0.08g/(g.h). By optimizing the process conditions, volumetric and specific productivity were increased to 0.09g/(L.h) and 0.15g/(g.h), respectively. Highest biomass concentration amounted to 16.8g/L, containing 73 % PHBHV,62 Using hydrolyzed whey permeate as main carbon substrate and valeric acid and γ-butyrolactone (GBL) as precursors for 3HV and 4-hydroxybutyrate (4HB), respectively, enabled the production of a high-quality poly(3HB-co-21.8%-3HV-co-5.1%-4HB) terpolyester. After recovery of the terpolyester and in-depth polymer characterization [DSC, molecular mass, molecular mass distribution], the authors suggested using the terpolyester for high-performance applications, e.g., in the medical field.48 Based on the high salinity needed to farm Hfx. mediterannei efficiently, the risk of microbial contamination is negligible; sterility precautions can be more or less neglected. Microbial contamination was not evidenced when cultivations were carried out over extended time periods even without any sterilization precautions.28 Economic assessment of Hfx. mediterannei-mediated PHA production on hydrolyzed whey and solvent-free downstream processing, based on experimental data from 200L scale,estimate the production price below € 3 per kg PHA.12
Hfx. mediterannei also constitutes a promising candidate for production of PHA co- and terpolyesters based on crude glycerol phase (GLP), a major by-product of the emerging biodiesel production. Hermann-Krauss et al. [28] reported the production of PHBHV with 10mol.-% 3HV at a volumetric productivity of 0.12g/[Lh] and 75% PHBHV in biomass. Molecular mass was determined with 150-253kDa, Pi of 2.1-2.7, and Tm between 130°C and 140°C in different production setups. Supplying the 4HB-precursor GBL generated a terpolyester containing 12mol-% 3HV 5mol-% 4HB (0.05mol/mol) with lower Tm (two melting endotherms at 122 and 137°C) and glass transition temperature (Tg 2.5°C) and higher molecular mass (391kDa).
Huang et al.50 used extruded rice bran (ERB) and extruded corn starch (ECS) as carbon sources for Hfx. mediterannei mediated PHA production without nitrogen limitation. Extrusion was needed due to the strain´s lacking ability to directly convert rice bran or cornstarch in its native form. By employing controlled pH-stat feeding strategy (pH-value between 6.9 and 7.1) in a 5L bioreactor using ERB/ECS mixtures (1/8g/g) as main carbon source in a repeated fed-batch feeding regime, a cell dry mass (CDM) concentration of 140g/L, a PHA concentration of 77.8g/L and a PHA content in CDM of 56wt.-% were obtained. Using ECS as sole carbon source, 62.6g/L CDM, 24.2g/L PHA concentration and 38.7wt.-% PHA in CDM were reached. Under the applied highly saline conditions, the authors suggest that it was possible to keep the repeated fed-batch process running for mass production of PHA for extended periods.
Corn starch, pre-treated by an enzymatic reactive extrusion process (single-screw extruder with α-amylase quantities at 1-5g/100g wet mass of cornstarch), was used for PHA production by Hfx. mediterannei by Chen and colleagues.51 A mixture of extruded starch and yeast extract was used in a ratio of 1/1.7g/g in the feeding medium to maintain carbon and nitrogen concentrations constant during the pH-stat fed-batch process. PHA content in CDM reached 50.8wt.-%. Also in this case, a PHBHV copolyester with 10.4mol-% 3HV, a Tg of -1.2°C, and with two melting peaks at 129.1°C and 144.0°C was produced.
Independent on the carbon sources, the high salinity of the Hfx. mediterannei medium of about 20-25wt.-% NaCl requires special bioreactor material and measuring sensors sustaining the high salinity; high-quality steel or polymers like PEEK were proposed.63 For reasons of both economics and sustainability, the possibility to recycle the highly saline side streams of this process was subjected towards investigation. Salt disposal after completion of the fermentation constitutes a problem as valid ecological standards do not permit the discharge of total dissolved solids (TDS) above 2,000mg/L in waste water. In this context, it was demonstrated that the spent fermentation broth from whey-based PHA-production by Hfx. mediterannei can be used to substitute a considerable share of fresh saline cultivation medium in next production cycles. Additionally, about 29% of yeast extract, an expensive growth additive needed as nitrogen-and phosphate source for efficient cultivation of the strain, can be replaced by cell debris from previous cultivations.64 Similar strategies were followed by Bhattacharyya and colleagues,65 who used waste stillage from the rice-based ethanol production for Hfx. mediterannei mediated PHB production. On a shaking flask scale, the authors reached about 70wt.-% PHA in CDM, a PHA concentration of about 16.4g/L, a PHA yield from the substrate of 0.35g/g, and a volumetric PHA productivity of 0.17g/(L h). Again, the produced PHA was a PHBHV copolyester with a 3HV fraction of 15.3 mol-%. A remarkable reduction of both the chemical and biochemical oxygen demand (COD and BOD) of stillage by about 85% was highlighted as the most beneficial outcome of the study. Also in this experiment, PHA was released from the cells by hypotonic cell lysis, and was further purified by organic solvents and sodium dodecyl sulfate (SDS). The TDS content in the final discharge water amount to merely 670mg/L, which is only about 30% of the allowed concentration.
Apart from Hfx. mediterannei, additional other haloarchaea were screed and isolated. From Indian brine and sediment samples of solar salterns, Salgaonkar et al.66 screened seven extremely halophilic archaeal isolates with the capability to growth and produce PHA in a synthetic medium containing 20% NaCl. Based on phenotypic and genotypic characterization, six of the isolates, termed TN4, TN5, TN6, TN7, TN10 and BBK2, were classified as Haloferax ssp.; the isolate TN9, on the contrary, was classified as a Halogeometricum borinquense. Growth and PHA accumulation kinetic studies revealed that Hgm. borinquense [TN9] displays maximal PHA productivity during the exponential growth phase, which classifies it as a ”growth-associated PHA-producer”; about 14wt.-% of the homopolyester PHB in CDM were achieved after 5 days of cultivation.
Singh67 isolated halotolerant and halophilic organisms from (agro)industrial surroundings, and cultivated the strains on different solid media containing 2M NaCl. Best growth occurred on solid Luria medium; also on selective media (DSC97), significant growth was observed. After optimizing the culture conditions, the organisms were characterized biochemically and by different staining techniques (Gram, Sudan Black, etc.). Based on 16S rRNA analysis, the isolates with the most promising PHA-production potential were identified as Gram-positive Bacillus subtilis ssp. and the Gram-negative Pseudomonas sp.
Danis et al.68 carried out similar experiments with five extremely halophilic archaeal isolates in order to trace the strain with the highest PHA-production capacity. PHA production of each isolate was individually examined on various inexpensive carbon sources such, e.g., sucrose, whey, corn starch, and waste of apples, melons and tomatoes. Among these feedstocks, corn starch turned out to be a promising substrate for PHA biosynthesis. Among the investigated strains, isolate 1KYS1 displayed the highest PHA biosynthesis capacity. Comparative 16S rRNA gene sequence analysis revealed the close relationship of 1KYS1 to the genus Natrinema, especially to the species Natrinema pallidum JCM 8980. On starch as sole carbon substrate, 1KYS1 reached a PHA content in CDM of about 53.14.-%. Large and uniform intracellular PHA granules were observed by transmission electron microscopy (TEM), and identified as the copolyester PHBHV. PHBHV produced by 1KYS1 was blended with poly(ethylene glycol)l of low molar mass for preparation of biocompatible films which can be used for drug release experiment with Rifampicin as model compound. Most efficient drug delivery was achieved at 37°C and a pH-value of 7.4.
Cyanobacteria form a group of photosynthetic nitrogen-fixing bacteria, which are present in almost all aquatic or terrestrial habitats, and are increasingly in the spotlight as third generation PHA producers.69,70 Shrivastav and colleagues71 studied the marine photoautotroph cyanobacterium Spirulina subsalsa, originally described as an isolated from samples from the Indian coast. This organism turned out to display enhanced PHA production when exposed to elevated salinity levels.
In addition, Halomonas campaniensis LS21, a halophile and in parallel alkalophile eubacterium, was isolated and cultivated in an open, non-sterile, continuously operated PHA production process. This process was based on alkaline seawater and artificial carbonaceous kitchen waste, predominately consisting of polysaccharides, lipids, and proteins. PHB was selected as model product of the strain during long-term cultivation to assess the general viability of open and long-term cultivation of industrially relevant bacteria, and to investigate genetic engineering of this organism to further enhance the process. Both the wild type strain and a recombinant H. campaniensis LS21, additionally equipped with the PHB synthesis genes phbCAB, were farmed in a continuous process for 65 days in artificial seawater-based medium. Under extremely saline (27g/L NaCl) and highly alkaline (pH-value 10) conditions and a moderate temperature of 37°C according to the strain´s optimum values, the genetically engineered strain achieved about 70wt.-% PHB in CDM, in contrast to 26 wt.-% achieved by the wild type organism. Despite the open process regime, both cultures remained monoseptic. Extracellular hydrolytic enzymes were excreted during the entire cultivation, which enabled them to convert the mixed substrates. Until the end of the cultivation, the plasmid carrying phbCAB genes maintained stable in the recombinant cells. Combined with its expedient susceptibility for genetic improvement, H. campaniensis LS21 definitely might constitute a powerful cellular factory for cost- and energy-efficient production of PHA or extremozymes from inexpensive feedstocks.72
In the context of alkaliphily, as described for H. campestris, also cyanobacterial strains were found which preferably accumulate PHA under elevate pH-values. This is especially the case for Spirulina platensis; for this organism, the optimum pH-value for both PHA production and degradation is reported in the rather strong alkaline range of 9 to 11.73
The halophile bacterium HalomonasTD01, isolated from a Chinese salt lake, was used by Tan and associates74 for a similar non-sterile, continuous cultivation process. In 56 h fed-batch fermentation set-ups based on glucose as the sole carbon source, the strain reached up to 80g/L CDM containing 80wt.-% PHA. In an open, non-sterile, continuous two-stage process operated for two weeks, CDM reached an average concentration of 40g/L with about 60wt.-% PHB in CDM in the first stage, which contained a saline medium rich in glucose and nitrogen. The fermentation broth was continuously transferred from the first to the second, nitrogen-free stage. Although this transfer diluted the CDM concentration, a constant PHB fraction in CDM between 65 and 70wt.-% was reached. In the first stage, between 0.20 and 0.30g PHB were generated per g converted glucose, but, surprisingly, more than 0.50 g per g in the second stage.
Halomonas TD01 was later subjected towards genetic engineering. By knocking out the 2-methylcitrate synthase encoding gene, propionate-to-3HV conversion efficiency was almost doubled, resulting in an increase of 3HV in random PHBHV copolyester of almost 100%. In a minimal medium containing glucose and 0.5g/L propionate, cells accumulated 70wt.-% PHBHV in CDM with a molar 3HV fraction of 12%. The effect of deleting the genes encoding for PHA depolymerase in order to prevent intracellular PHA degradation, especially in later stages of large-scale cultivations, was also investigated, but the overall PHA-output was not significantly enhanced. In 500L pilot-scale cultivation studies, CDM of genetically engineered Halomonas TD01 reached 112g/L, with 70wt.-% PHB in CDM on glucose as the sole carbon source; in the presence of propionate, 80g/L CDM with 70wt.-% PHBHV (8mol-% 3HV in PHBHV) were obtained. On shaking flask scale, PHB mass fractions of even 92wt.% and significantly enhanced glucose-to-PHA conversion were achieved. Further experiments to increase stability of genetically engineered Halomonas TD01 encompassed the partial inhibition of the DNA restriction/methylation system, and, in order to induce the expression of multiple pathway genes, the construction of a stable conjugative plasmid pSEVA341. Together with the above described insertion of 2-methylcitrate synthase and knockout of depolymerase, the new construct HalomonasTD08 was able to accumulate up to 82wt.-% PHBHV in CDM.75 Stability and performance of all these promising genetically engineered halophiles should now be tested for long-term stability under continuous operation.
Han et al.76 were the first who experimentally investigated the genes responsible for archaeal PHA synthesis. The authors revealed that the haloarchaeon Haloarcula marismortui, isolated from the Dead Sea, was able to accumulate up to 21wt.-% PHB in CDM in minimal medium containing excessive glucose, and identified the adjacent genes phaEHm and phaCHm which encode two subunits of a Class-III PHA synthase and are directed by a single promoter upstream of the transcriptional start site; the genes were constitutively expressed under both nutritionally balanced and nutrient-limited conditions. Remarkably, PhaCHm turned out to be, in contrast to PhaEHm, strongly connected to the PHA granules. The introduction of phaEHm or phaCHm into Haloarcula hispanica, a strain harboring highly homologous phaECHh genes and widely used in haloarchaeal studies, particularly for isolating haloviruses, boosted PHB synthesis in this recombinant organism; coexpression of both genes resulted in highest PHB production. It is worth to mnetion that the knockout of phaECHh genes in H. hispanica resulted in a total termination of PHA synthesis. PHA accumulation capability and PHA synthase activity was successfully restored by complementation with phaECHm genes. These outcomes demonstrated the importance of phaEC genes for PHA accumulation in Archaea. Later, Ding et al.77 carried out the sequencing of the genome of a H. hispanica ssp., and observed significant differences from the published gene sequence of the model strain H. hispanica ATCC 33960.
Estuaries and coastal marine sites are examples of saline environments rich in halophilic microbes. Lau et al.78 reported the gene sequence of a Yangia sp. CCB-MM3, a halophilic bacteria isolated from Malaysian Matang Mangrove soil sediments, as the first completely deciphered gene sequence of a Yangia sp., which are members of the Rhodobacteraceae family. This ecosystem is influenced by both marine conditions and by freshwater flow. The genome consists of two chromosomes plus five plasmids , has a total size of 5.522,061bp with 65% GC content in average. Genome sequence analysis confirmed the presence of a propionyl-CoA synthesis pathway and of a gene cluster for PHA production in this strain. The ability of Yangia sp. CCB-MM3 for PHA accumulation was tested and confirmed in vitro. The authors suppose that the genome sequence of this strain will considerably contribute to the understanding of the physiology and metabolism of Yangia sp., and should be compared with the genome of other and new members of the Rhodobacteraceae family.
Gram-positive halophile PHA producers: Bacillus megaterium uyuni S29 represents the still rather scarce number of described Gram-positive organisms which produce significant amounts of PHA in saline enviroments. This strain, originally isolated from the Uyuni salt lake in Bolivia,79 features high capacity of PHB production in typical nutrient media used in industrial biotechnology.80 The impact of fluctuating salinity on growth and PHB production by this strain was assessed under different NaCl concentrations selected according to the salinities dominant in the strain’s natural salt lake environment.81 The strain displayed astounding adaptability to fluctuating salinity, with best results for growth and PHB accumulation at 45g/L NaCl. The strain still grows well at salinities as high as 100g/L NaCl, PHB production was detected even at 25wt.-% NaCl. As another benefit, no sporulation, which typically restricts the application of Gram-positive strains for PHA production due to the loss of the carbon flux, was observed in all experiments. Like all Gram-positive strains, this organism does not produce inflammatory active endotoxins [lipopolysaccharides, LPS], which impedes the in vivo application of its PHA in the medical field. Overall, this strain appears appealing not only for production of PHA for biomedical purposes, but also for other biotechnological process such as saline wastewater treatment.
Tracing halophile PHA producers: Numerous techniques for tracing PHA in various different microbes were described in the past.82 In the case of halophiles, existing PHA detection methods are not precise enough, frequently deliver false positive results, and/or need prior PHA synthesis before the screening. To overcome this shortcoming, Mahansaria et al.83 developed a new method based on amplifying the conserved gene region phaC, encoding for the Class-III PHA synthase of halophiles. This region is about 280-300bp in size. For the amplification, the primers codehopCF and codehopCR, developed by Han et al.,84 were used. The new method was tested with in total nine known haloarchaeal and halobacterial strains, and 28 own halophilic isolates from the Indian coast. Twenty-nine strains were phaC-positive, eight phaC-negative despite positive results from previous Nile Red staining. 16S rRNA analysis identified nine novel haloarchaea and nine novel halobacteria as PHA producers. Based on multiple sequence alignment of the phaC gene-derived amino acid sequences, it was shown that only seven amino acid residues were conserved within all four synthase classes encoded by phaC, whereas 61 amino acids were identical among the synthase encoded by the phaC specific to the investigated halophiles. All phaC-positive strains produced PHA in nutrient-limited medium, whereas no PHA production was evidenced by the phaC-negative strains. The authors evaluated their new method as highly precise, because it enables eliminating false positive results from Nile Red staining, e.g., due to the accumulation of lipophilic inclusion products different to PHA.83
Cryophilic [Psychrophilic] PHA producers
General aspects about cryophilic organisms: Low temperature is a critical factor in sustaining cell division and survival of organism. Reduced protein synthesis, cold-denaturation of proteins, decreased membrane fluidity, low cross-membrane diffusion rates, formation of ice crystals, and production of noxious highly reactive oxygen species are typical results of the exposure to low temperature. Frequently, microbes living in cold environments are exposed to several stress factors in parallel. Deep-sea organisms, for example, have to endure low temperature and high pressure conditions, while microbes in polar regions usually have to tolerate low temperature, nutrient deficit, and increased UV-radiation. Consequently, microbes resistant to multiple stress factors are to be selected in such extreme environments.85 A range of different sea-ice bacteria populate small brine channels and have developed a biofilm-like organization, characterized by high cell densities and EPS excretion.86 Substrate disposal and low temperature act as cooperative growth-restricting factors; nevertheless, some microbes are still able to grow in cold, even frozen water, unless both substrate availability and temperature reach a critical level at the same time.87 Although sea ice is colder than the seawater underneath, the concentrations of (macro)nutrients and convertible carbon sources can be higher, thus enabling sea-ice microbes to grow and probably to store carbon and energy as PHA granules.87 Presence of PHA increases the fitness of the microbes under such changing environmental conditions.
Reports on PHA production by psychrohilic microorganisms: Ayub et al.88 have reported that phaRBAC genes from Pseudomonas sp. 14-3, a Gram-negative, mobile, rod-shaped, non-spore-forming Antarctic bacterium, are located on an isolated gene region, which harbors other genes, which most likely contribute to its adaptability to low temperature. Later, the same researchers studied the effect of PHA accumulation on the adaptability of Pseudomonas sp. 14-3 to low temperature by direct comparison to its PHA synthase-minus mutant, obtained by deleting the phaC gene encoding for the PHA synthase. The PHA-negative mutant was more prone to freezing than its parent strain, and did not grow at 10°C. It was assumed that PHA-reserves are pivotal to develop the oxidative stress response provoked by low temperature. It was demonstrated that the cold-sensitive phenotype of the phaC-negative mutant can be suppressed by adding reduced compounds [cysteine, gluthathione]. Further, a sudden cold shock provoked very rapid PHA degradation and a drastic decrease of the NADH/NAD ratio and NADPH content in the PHA-positive strain. Consequently, lipid peroxidation in the mutant strain was 25-fold higher after decrease of temperature. Hence, PHA metabolism controls the availability of the reducing equivalents NADH and NADPH, thus alleviating the oxidative stress caused by cold temperature, by supplying the reductive power necessary to subdue the oxidative stress induced by cold conditions via PHB degradation.89
The same organism, Pseudomonas sp. 14-3, was characterized by López et al.90 by 16S rRNA gene sequence analysis as well as by physiological and biochemical tests. It was revealed that the strain belongs to the genus Pseudomonas sensu stricto, and displays 99.7% sequence similarity to Pseudomonas veronii DSM 11331T, although DNA–DNA hybridization experiments between the two strains reveled only modest re-association similarity. The bacterium grew in a temperature range from 4 to 37°C, but not at moderately thermophile conditions of 42°C. When cultivated on sodium octanoate, it accumulated the homopolyester PHB. The authors proposed to classify the organism as the type strain of the new species entitled Pseudomonas extremaustralis sp. nov. Tribelli et al.91 carried out further experiments with this strain and its PHA-negative mutant by investigating the relationship between its PHA accumulation and EPS production at low temperature in shaking flasks cultures or in cultures emedded in poly (styrene) biofilm microplates. Microaerobic growth, EPS production and motility were studied. It turned out that, under cold conditions, PHA accumulation goes in parallel with increased motility and supports the survival of planktonic cells in the developed biofilms. Microaerobic conditions, to a certain extent, saved the cold growth deficiency of the PHA-negative mutant. The authors assumed that the capability for PHB accumulation might display an adaptive advantage for the settlement in new, environmentally stressful ecological niches. Later, the same authors sequenced the entire genome of the strain as in order to identify all genes responsible for PHA metabolism, adaptability to extreme environments, and degradation of toxic compounds. As a result, the first completely sequenced genome of a psychrophilic organism is reported.91 Further, they investigated the strain´s potential for bioremediation, specifically for degradation of the eco-pollutant diesel. Such bioremediation requires the use of micobes capable to adapt to extreme environments contaminated with toxic compounds. Different cultivation conditions for P. extremaustralis, namely embedding the cells in biofilm (benthic cultivation), or as planktonic cells in agitated shaking flask cultures, were applied. Benthic cultures, hence, those protected by biofilms, displayed better growth, more biosurfactant production and higher diesel degradation, if compared with the planktonic cultures. PHA accumulation decreased the attachment of biofilm cell and enhanced the production of biosurfactants. Long-chain and branched alkanes were degraded in biofilms, while free cells degraded only medium-chain length alkanes. These outcomes suggest the cryophilic PHA-producer P. extremaustralis as an excellent candidate for hydrocarbon bioremediation in extreme environments.92
Ciesielski et al.93 searched for additional cryophilic microbial PHA producers in freshwater reservoirs of the Antarctic Ecology Glacier foreland. 16S rRNA gene sequence of isolated strains enabled their classification as members of the classes of α-, β-, or γ-proteobacteria, followed by detecting the individual PHA synthase genes via PCR. Potential PHA producers were isolated from all investigated sampling sites; predominantely, the organisms belonged to the genera Pseudomonas and Janthinobacterium. This report on the frequent occurrence of PHA producers in such cold environments was a further evidence that PHA synthesis occurs as a common feature of pioneering microbes. The isolates belonging to the genus Pseudomonas had the genetic potential to synthesize medium-chain-length PHA (mcl-PHA; evidenced by the presence of phaC1 genes), whereas some new isolates of Janthinobacterium might produce either short-chain-length PHA (scl-PHA) or mcl-PHA (evidenced by presence of both phaC and phaC1 genes) as the first report revealing mcl-PHA production by Janthinobacterium sp. The authors also observed the lacking correspondence between the evolutionary history of the 16S rDNA genes and those coding for PHA synthases of the isolated Janthinobacterium sp. strains. Based on phylogenetic analyses of phaC and phaC1 the authors assume that these genes could have entered the genomic material of Janthinobacterium sp. via horizontal gene transfer, and assumed that presence of these PHA genes may increase bacterial fitness for survival under environmentally harsh conditions.
In order to better understand the role of PHA production in environment characterized by both low temperature and high salinity, a long-term study spanning over a 10-years period was accomplished by Pärnänen et al.94 to search for PHA producers in the sea ice, the cold seawater, and in the cold estuarine of the northern Baltic Sea, more specifically on the Nord-Eastern coast of Sweden, and the Western and South western Coast of Finland. Samples collected in these extreme environments were scrutinized for PHA producers by Nile Blue A staining of microbial inclusions, and by PCR assays to detect PHA-synthase-encoding genes. Both PHA granules and PHA synthase genes were found in sea water and ice at all sampling sites. By phaC gene sequencing, high similarities of the isolates with the species Rhodoferax ferrireducens, Polaromonas naphthalenivorans and Polaromonas sp. [β-proteobacteria], and Pseudomonas extremaustralis strain 14-3 [γ-proteobacterium] was evidenced. The study proofed for the first time PHA synthesis by microbes adapted to the environmental conditions of the Baltic Sea, making this region an interesting spot in the search for new PHA producers.
Cryo scanning electron microscopy (cryo-SEM) provides a modern tool to investigate the influence of intracellular PHA granules on the cell´s mechanical properties, mobility of cytoplasmic solutes, and the cell´s reaction to temperature changes, e.g., during freezing, towing, or drying.95 Using this tool, Obruca and associates 96 revealed the cryo-protective effect of PHB in bacterial survival under freezing conditions on a mechanistic basis. PHB´s monomer 3HB was recognized as a powerful cryo-protectant for lipase, yeasts, and the PHB-producing bacterium C. necator during freezing-thawing cycles. Viability of frozen–thawed PHB-positive C. necator cultures was drastically increased if compared to that of the PHB-negative mutant cultures. Presence of intracellular PHB granules featured a significant advantage during freezing; the authors assumed that this effect is partly due to the high levels of 3HB in strains capable of simultaneous PHB synthesis and mobilization, as it is the case in C. necator cyclic PHB metabolism. Apart from the benefical role of 3HB, the cryo-protective mechanisms of PHB granules seem to be more complex, as detected by cryo-SEM observations: even at extremely low temperatures, intracellular PHB granules preserve their high flexibility, which make it likely that they prevent cellular damage by intracellular ice crystals, such as physical membrane damage, gas bubble formation, or disruption of organelles. The presence of PHB granules seems to change the adhesive forces between water molecules and cellular components; water in PHB-containing cells obviously can be more easily released from the cell during drying or freezing by a higher rate of transmembrane water transport, which prevents ice formation.
Thermophile PHA producers
General aspects about thermophiles: Cultivations in bioreactors carried out with thermophilic microorganisms are considered energy-efficient, because only little cooling efforts are required. In addition, thermophilic fermentations are “self-heating” due to the heat energy generated by the microbes’ metabolic activity, as especially noticed at cultivations with high biomass concentration. In addition, the bioreactor´s stirring system generates heat energy, which can also be provided to the fermentation process. These multiple effects enable the lowering of both heating and cooling costs. As an additional benefit in contrast to the application of mesophilic bacteria, sterility precautions may be saved when culturing thermophilic organisms, which is similar to the advantages described above for halophile organisms.29
Mechanistic consideration about the interrelation between high temperature and the metabolic PHB cycle were recently carried out by Obruca and colleagues,97 who revealed the role of the monomer 3HB as a chemical chaperone protecting several enzymes like lipase and lysozyme from denaturation by high temperature and oxidation. High temperature-mediated lipase denaturation in the presence or absence of 3HB was monitored, and showed a significant protective effect of PHB; this effect was even higher than displayed by common chaperones like trehalose and hydroxyectoine. PHB was able to protect lipase not only against heat-mediated denaturation but also against oxidative damage by Cu2+ and H2O2; its protection was higher than that of trehalose and comparable to that of hydroxyectoine. These results confirm previous reports by Soto et al.41 who suggested the role of PHA in preventing enzyme denaturation under extreme salinity and temperature conditions.
Gram-negative thermophile PHA producers: Pantazaki et al.98 were the first to study PHA biosynthesis by the thermophilic bacterium Thermus thermophilus. At outstandingly high temperatures up to 75°C, the authors used sodium gluconate or sodium octanoate as sole carbon sources, and reached PHA contents in CDM of about 35-40wt.-%. The two substrates resulted in completely different types of PHA in terms of their monomeric composition. Whereas cultivation on gluconate resulted in a copolyester consisting mainly (about 64mol-%) of 3-hydroxydecanoate (3HD)and smaller fractions of 3-hydroxyoctanoate (3HO), 3HV, and 3HB, whereas analysis of the PHA obtained by cultivation on octanoate revelead the composition of this copolyester as follows: 3HB 24.5mol-%, 3HO 5.4mol-%, 3-hydroxynonanoate (3HN) 12.3mol-%, 3HD 14.6mol-%, 3-hydroxyundecanoate (3HUD) 35.4mol-%, and 3-hydroxydodecanoate (3HDD) 7.8mol-%. The first copolyester (based on gluconate) had a weight average molecular mass of Mw=480,000g/mol, whereas Mw of the second polymer (based on octanoate) amounted to 391,000g/mol. Activities of enzymes pivotal for PHA biosynthesis, namely 3-ketothiolase, NADPH-dependent reductase, and PHA synthase were measured in the soluble cytosolic fraction obtained from T. thermophilus cells grown on gluconate. PHA synthase from gluconate-grown cells was isolated, purified, and characterized, revealing temperature and pH optimum for its enzymatic activity of about 70°C and pH 7.3, respectively. Free CoA and alkaline phosphatase completely inhibited PHA synthase activity. In contrary to other known PHA synthases, kinetics of this enzyme did not exhibit any lag phase. The authors provide further predicted an important role of cysteine in the catalytic mechanisms of the synthase as the first purified and characterized thermophilic PHA synthase. In a later study, the authors used PCR-based methods to identify the PHA biosynthesis genes in the strain´s genome. The experiments revealed that T. thermophilus HB8 belongs to PHA producers harboring Class-II PHA synthases.99
In the field of phototrophic thermophiles, Hai et al.100 screened 11 different cyanobacteria for their capacity of PHA production, and determined the type of involved PHA synthase. For the investigations, Southern blot analysis using a phaC-specific DNA probe, Western blot analysis using specific polyclonal anti-PhaE antibodies raised in this study against PhaE of Synechocystis sp. strain PCC 6803, PCR sequence analysis, and sequence analysis of PHA synthase structural genes, were used. These methods revealed the presence of a Class-III PHA synthase in the cyanobacterial species Anabaena cylindrica SAG 1403-2, Chlorogloeopsis fritschii PCC 6912, Cyanothece sp. strains PCC 7424, PCC 8303 and PCC 8801, Gloeocapsa sp. strain PCC 7428, and Synechococcus sp. strains MA19 and PCC 6715, whereas no Class-III synthase genes were found in Cyanothece sp. strain PCC 8955, Gloeothece sp. strain PCC 6501, and Stanieria sp. strain PCC 7437. Protein extracts and chromosomal DNA of Synechocystis sp. strain PCC 6803, the first cyanobacterium with completely available PHA synthase genes, acted as positive control. The entire PHA synthase structural genes of Chlorogloeopsis fritschii PCC 6912 and Synechococcus sp. strain MA19 and a central region of the Cyanothece sp. strain PCC 8303 phaC gene of, were cloned, sequenced, and transferred into Escherichia coli for heterologous expression.
Synechococcus sp. MA19, a thermophilic cyanobacterium known for photoautotrophic production of high amounts of PHB, was isolated from the surface of a Japanese volcanic rock, as originally described by Miyake and colleagues.101 This strain grows best at 50°C, and, when cultivated photoautotrophically in simple bottles by a submerged aeration stream enriched with 2% CO2, can accumulate up to 21wt.-% PHB in CDM. This was the first report suggesting a cyanobacterial strain as serious candidate for PHA production on larger scale. Careful fine-tuning of CO2 supply and illumination are decisive to improve PHA productivity by this strain, requiring an optimized process engineering for large-scale implementation. In nitrogen-free dark cultivation setups, it was possible to boost the PHB content in CDM to even 27wt.-%; this was based on the degradation of a second storage compound of this strain, glycogen, under dark conditions. Once accumulated, intracellular PHB was only degraded in nitrogen-rich conditions under illumination; no PHB degradation occurred in the dark, neither with nor without exogenous nitrogen.
In addition to nitrogen-limited cultivation conditions, further efforts to boost PHA production in thermophile cyanobacteria were undertaken by Nishioka et al.,102 who cultivated the organism under phosphate-depriviation. Under autotrophic conditions and phosphate limitation at 50°C, PHB production at intracellular phosphate levels between 0.043 and 0.076mmol per g CDM was investigated in Spirulina sp. MA19. 55wt.-% PHB in CDM, 2.4g/L PHB and 4.4g/L CDM were achieved in a 260 h old culture containing Ca3(PO4)2 as insoluble phosphate source, which was almost the two-fold output in comparison with setups well supplied with phosphate. Based on the results, the authors suggested to operate fedbatch cultures with this organisms in such a way to prevent phosphate concentration to exceed the intracellular phosphate level.
In the field of chemoorganotroph extremophile PHA producers, Chen et al.103 isolated an amylase-excreting strain from a hot spring located in Pingtung, Taiwan, and labeled it as isolate On1T. Its cells were Gram-negative, monotrich flagellated rods. Temperature optimum for growth was determined with about 55 °C, together with a neutral pH optimum, whereas 16S rRNA gene sequence analysis classified it as a member of the phylogenetic class of β-proteobacteria. Based on 16S rDNA sequences, DNA– DNA similarity, physiological and biochemical particularities, and its fatty acid pattern, the strain was determined as a new species in the genus Caldimonas. The authors proposed its classification as Caldimonas taiwanensis sp. Nov. Later, Sheu et al.104 detected that, under nitrogen-limited conditions, C. taiwanensis produces PHB at 55°C from common carbon sources such as fructose, gluconate, glycerol, and maltose, but not from fatty acids if provided as sole carbon source. PHBHV copolyester production occurred by co-feeding a mix of gluconate and valerate. Depending on the valerate concentration, the molar3HV fraction was triggered from 10 to 95mol-%. These outcomes are coherent with the strain Caldimonas manganoxidans previously described as PHA producer at a temperature optimum of about 50°C by Takeda et al.105 Similar to other PHA producing strains such as Burkholderia funghorum (previously Pseudomonas hydrogenovora),106 valerate substantially inhibited growth and PHA synthesis already at levels as low as 5mM in Sheu´s experiments with C. taiwanensis. Yeast extract supplementation elevated the inhibiting effect, and enhanced both biomass yield and PHA productivity. In vivo tests with isolated C. taiwanensis PHA synthase showed its substrate specificity for 3HB, 3HV, and 3-hydroxyhexanoate (3HHx). Based on the strain´s amylase activity, cultivations were also performed on mixtures of soluble starch from cassava, corn, potato, sweet potato or wheat as main carbon source and valerate as 3HV precursor, again resulting in the production of PHBHV copolyesters with different 3HV fraction in dependence on the starch/valerate ratio in the substrate feed. Based on the conversion of inexpensive starch as carbon source and the reduced cooling costs by the thermophilic process regime, the authors underline the promise of this strain for cost-efficient PHBHV production.
Ibrahim et al.107 isolated and screened a range of thermophilic microbes able to convert inexpensive carbon feedstocks to PHA under elevated temperature conditions. The microbes were enriched from a German aerobic waste treatment plant, and from Egyptian hot springs. Nile Red staining of colonies grown on solid agar plates with different sole carbon sources resulted in the isolation of six Gram-negative bacteria. During growth, five of these strains, labeled MW9, MW11, MW12, MW13 and MW14, were organized in stable star-shaped cell aggregates [SSCAs], whereas strain MW10 occurred as a consortium of individual planktonic rods. 16S rRNA gene sequence analysis revealed all of them as members of the class of α-proteobacteria. All isolates reveled 16S rRNA gene sequence similarity of more than 99%, and highest similarities between 93 to 99% with Bosea minatitlanensis, Bosea thiooxidans, Chelatococcus asaccharovorans, Chelatococcus daeguensis, Chelatococcus sambhunathii, and Methylobacterium lusitanum. MW9, MW10, MW13 and MW14 preferred glucose as carbon source, whereas MW11 and MW12 grew best and produced most PHB on glycerol. Highest attained values for CDM concentration and PHB fraction in CDM were 4.8g/L and 73wt.-%, respectively. All strains grew between 37 and 55°C, with 50 °C as the optimum growth temperature. The authors highlighted their study as the first report on SSCA formation as well by thermophilic as by PHA-accumulating strains, and emphasize again the high economic potential of PHA-producing thermophiles in terms of process cost reduction. The authors characterized strain MW9 by 16S rRNA gene sequence analysis and suggested its relationship to the genus Chelatococcus. Later, rep-PCR genomic fingerprints, fatty acid pattern, G+C content of DNA, and partial dnaK gene sequence confirmed this assumption, but also revealed that these isolates differ from other described Chelatococcus sp. DNA-DNA hybridization with other Chelatococcus sp. revealed degrees of relation between 11.0 and 47.7%. The authors therefore suggested the classification of the strain MW9 as a novel Chelatococcus sp., and proposed the name Chelatococcus thermostellatus for this powerful candidate for PHA production at elevated temperature.
A second isolate of Ibrahim´s series,29 Chelatococcus sp. strain MW10, was subjected by the same group of scientists towards experiments aiming at increasing CDM by advanced cultivation techniques. In a fedbatch fermentation on a 2L bioreactor scale, excess glucose above 20g/L was provided by pulse feedings to boost both biomass and PHB production, and to avoid intracellular PHB degradation. Maximum CDM and PHB concentration was obtained after 53h with 5.2±0.6g/L and 2.9±0.7g/L, respectively. Unfortunately, in the later phase of the process, PHB content drastically decreased, despite the permanent availability of carbon source. Therefore, a cyclic batch fermentation (CBF) was carried out on a 42L bioreactor scale at 50°C and 50g/L gluocse. Cycle time (50-h cultivation batches) was chosen according to the results from the the 2L fedbatch setup. The cultivation process was initiated with a volume of 25L; pO2 was controlled permanently at 20% saturation by adjusting the stirrer speed and the aeration rate. During the first cycle, high growth (μmax.=0.125/h) and significantly increased CDM up to 12.7±0.9g/L was reached, whereas PHB concentration (55.0±5.7wt.-%) was comparable to the value from the fedbatch setup. Now, 23 L of the fermentation broth were withdrawn, and replaced by the same volume of fresh, not sterilized medium. Remaining fermentation broth in the bioreactor acted as inoculum for the next cultivation cycle. Similar CDM of about 11g/L, but lower PHB contents in CDM (38.5±6.4wt.-% and 32.5±3.0wt.-%) were reached after the second and third cycle run, respectively., despite the permanent presence of glucose. As further process improvement, cyclic fed-batch fermentation (CFBF) was carried out as a modified semi-continuous culture technique, again on a 42L scale. Here, different volumes of the fermentation broth were replaced by fresh medium, in that way partly recycling 20 to 40% of the fermentation broth´s volume. Culture cycling was done at different intervals, considering the volume increase due to the semicontinuous feeding, and also the reduced pO2 level which occurred during the high cell density fedbatch process. In principle, adequate concentrations of glucose and ammonium were chosen to reach high CDM concentration and high PHB fractions in biomass. The CFBF started as a batch process with 30g/L glucose. Feeding of fresh medium was initiated after 21h of growth. The first cycling was performed after 44h of cultivation, when the cells grew relatively fast with a μ of 0.0701/h and well before PHB degradation had to be expected. Five L of the fermentation broth was replaced by the same volume of fresh medium. Subsequently, continuous feeding was carried out, followed by subsequent medium replacement cycles according to the need to minimize the volume increase. In order to prevent excessive dilution of the culture broth, the second cycle was completed by withdrawing 10L of culture volume and replacing by only 5L of fresh medium. After 14h in the third cycle, a final refilling of 5L of fresh medium was accomplished. Highest PHB contents exceeding 50wt.-% were obtained between 82h and 143 h during cycle 2. After finishing this cycle after 181h, 43.0±1.4g/L CDM, a polymer fraction in CDM of 39.0±8.5wt.-%, and the maximal PHB productivity of 16.8±4.2g/L were achieved. Cell growth increased drastically at the end of cycle 3, when a cell concentration of 115.0±4.3g/L was monitored. Although lower PHB fraction in CDM of 11.8±3.8wt.-% was noticed at the end of this process, a promising volumetric PHB productivity of 13.7±4.9g/L was achieved.
In 2014, the thermophile Chelatococcus daeguensis TAD1, a strain closely related to Chelatococcus sp. strain MW10, was isolated by Xu et al.108 from a biological mat of a biotrickling filter designated for NOx removal, and extensively investigated and compared to other PHB-producing microbes. This organism is a thermophilic PHB-producing bacterium. C. daeguensis TAD1 produced PHA in a growth-associated way without nutrient deprivation; within only 24h of cultivation at 45°C, the strain accumulated PHB up to 83.6wt.-% of CDM from glucose as the sole carbon source. As a remarkable outcome, C. daeguensis TAD1 displayed high tolerance to heat stress as well as to high nitrogen loads during PHB accumulation. On glucose, the highest PHB concentration, 3.44±0.3g/L, occurred at 50°C and a molar C/N-ration of 1/30. Further, the strain could efficiently convert various inexpensive carbon sources to PHB, such as glycerol and non-hydrolyzed starch. Using glycerol, the highest product yield (0.26 g PHB per gram converted glycerol) as well as PHB fraction in CDM (80.4wt.-%) were obtained. As a consequence, the authors suggest also C. daeguensis TAD1 as a viable production strain for industrial-scale PHB production on inexpensive feedstocks. These results were further improved by the same research group, who reported on the possibility of further increasing C. daeguensis TAD1-mediated PHB productivity on glycerol by a fed-batch cultivation regime. The authors discovered that PHB accumulation is inhibited by excess glycerol, but found out the beneficial effect of organic nitrogen sources like tryptone or yeast extract to boost growth of this strain. In batch fermentation setups, the application of low glycerol concentrations together with the supply of a nitrogen source cocktail consisting of NH4Cl, tryptone, and yeast extract, significantly promoted PHB biosynthesis by C. daeguensis TAD1. Based on these batch-results, fedbatch and two-stage fedbatch cultivation techniques were developed. In the two-stage fedbatch process, best results for PHB concentration (17.4g/L), corresponding to a volumetric productivity of 0.434g/(L.h), which is the currently highest PHA productivity reported for extremophiles, were obtained.109
Gram-positive thermophile PHA producers: In contrast to the huge number of reports on PHA production by Gram-negative bacteria and archaea, investigation of Gram-positive microbes able to produce PHA efficiently is still manageable, especially regarding the small number of reports on PHA production by extremophile Gram-positive strains.81 However, PHA produced by Gram-positives is characterized by low levels of LPS causing immunogenic reactions, making them interesting for biomedical applications. The first report on a thermophilic Gram-positive PHA producer was provided by Liu et al.,110 who describe the PHA accumulation by the bacterial isolate K5, which displayed excellent growth and PHB-accumulating capacity. Strain K5 has been isolated from the biofilm of a biotrickling filter designed for the removal of NOx from flue gas of a Chinese coal-fired power plant. Based on 16S rRNA gene sequence analysis and physiological and biochemical characterization, strain K5 has has turned out as a subspecies of Bacillus shackletonii, a species only scarcely found in literature. B. shackletonii K5 accepted glucose as carbon source for PHB synthesis at meso- and thermophilic temperatures between 35 to 50°C, with the temperature optimum determined at 45°C. Using glucose on shaking flask scale, the strain accumulated up to 69.9wt.-% PHB in 2.28g/L CDM. Employing succinate or glycerol as carbon source, 56.8 and 52.3wt.-%, respectively, of PHB in CDM were obtained. In batch-mode cultivations at 45°C and pH 7.0, B. shackletonii K5 produced PHB of up to 72.6wt.-% of CDM, which amounted to 9.76g/L on glucose as sole carbon source.
A further screening of thermophilic, contamination-resistant PHA producers was carried out by Xiao et al.111 During this study, the strain XH2, isolated from an oilfield, was characterized from a morphological, physiological, and biochemical perspective, and, supported by 16S rDNA analysis, classified as Aneurinibacillus sp. Nile red staining and transmission electron microscopy (TEM) reveled PHA granule accumulation by this strain, which was the first evidence for PHA production by a thermophilic representative of the phylum Firmicutes. By batch cultivation on glucose, peptone, and yeast extract at 55°C, 111.6mg/L PHA were produced. The PHA output was more than doubled in peptone-free medium. The produced PHA product mainly consisted of 3HB and 3HV and minor fractions of 3HO and 3-hydroxy-4-phenylbutanoate, as evidenced by GC-MS and NMR.
Giedraitytė et al.112 detected PHB production by the thermophilic organism Geobacillus sp. AY 946034, which can be cultured at temperatures up to 60°C. Under phosphate-limited conditions and glucose acting as the sole carbon source, the highest PHB fraction of 68.9wt.-% of CDM was observed. Based on the high monitored activities of 3-ketothiolase, CoA reductase and PHA synthase, hence, the enzymes responsible for PHB biosynthesis, the authors assumed that this organism uses the classical PHB biosynthesis pathway. FT-IR (characterization of chemical composition), DSC (thermoanalysis) and viscosimetry (molecular mass determination) were carried out by PHB characterization. FT-IR analysis identified the polyester as the homopolyester PHB. DSC reveled the semi-crystalline character of the polymer (Xc of 42%), an onset temperature of thermal decomposition (Td) of 280°C, Tm of 169°C, and a melting enthalpy (Hm) of about 63J/g. Molecular mass determination reveled a Mw of 556 kDa.
For rapid and efficient in vitro PHA production, thermostable enzymes are required. Tajima et al.113 reported PHA in vitro synthesis by the engineered thermostable PHA synthase PhaC1SG (STQK). In this system, however, also non-thermostable enzymes from mesophilic organisms, namely acetyl-CoA synthetase (ACS) and CoA transferase (CT), were used to biosynthesize the monomers. Therefore, the authors cloned ACS from Pelotomaculum thermopropionicum JCM10971 and CT from Thermus thermophilus JCM10941, both thermophilic organisms, in order to design a completely thermostable in vitro PHA synthesis system. Both enzymes displayed high thermostability, with temperature optima around 60°C and 75°C, respectively. In vitro PHA synthesis at 45°C was successfully accomplished by the collaborative action of ACSPt, CTTt, and PhaC1SG (STQK). In addition, yields of PHB and poly(lactate-co-3HB) were even higher at 37°C than those obtained in vitro synthesis system resorting to non-thermostable ACS and CT from mesophilic organisms.
Only recently, a comprehensive study by Levett et al.114 addresses the lack of abundantly available, inexpensive feedstocks as the major obstacle impeding large-scale PHA production.114 Methane´s abundant availability and its rather low market price qualifies this simple alkane it as a potential substrate for PHA production.115 Therefore, Levett and colleagues114 presented a comprehensive techno-economic analysis of methane-based PHA production using the process optimization software ASPEN Plus for process design and simulation. An annual production of 100,000 t PHB by methanotrophic fermentation, and extractive polymer recovery using a system of acetone and water, was considered. Production costs per kg PHA were tentatively calculated with US-$ 4.1-6.8, which is in the same range as the production price obtained by other studies. Compared with sugar-based PHA production, raw material costs are reduced from 30-50% of the entire production costs116 to only 22% when using methane as feedstock. Importantly, the authors revealed that heat removal from the two-stage bioreactor process contributes 28% of total operating cost. Thermophilic methanotrophs could enable using cooling water instead of refrigerants, which further reduces production costs per kg PHA to US-$ 3.2-5.4, which already approximates the optimistic estimations for whey-based PHA production by halophiles.12 Because energy needed for air compression and drying of biomass prior to PHA extraction were also spotted as significant cost factors, it is evident that bioreactor geometry and operation conditions, as well as biomass drying techniques should be optimized. Further, understanding the mass transfer of methane at high biomass concentration and elevated pressure is needed to support the economic optimization, together with a reduction of the acetone loss during polymer extraction. Most importantly, it has to be emphasized that these are in silico calculated estimations based on fictive model organisms; the quest for thermophilic methanotrophs with high PHA production potential is still in progress.
PHA production under heavy metal contamination and other stress factors
Pioneering work in the investigation of diverse stress factors like oxidants, organic solvents or freezing in triggering PHA synthesis, and in elucidating the protective role of PHA to sustain these stress factors were done in the recent years by a research group in Brno, Czech Republic. In the first of their experiments, Obruca et al.117 added diverse alcohols to cultivations of C. necator H16 on waste rapeseed oil as main carbon source for PHA accumulation. The authors noticed that especially the supplementation of 1-propanol after 24h fedbatch cultivation increased both biomass growth (from ca. 11 to ca. 15g/L CDM) and PHA biosynthesis, which doubled from about 6g/L in control experiments without alcohol addition to about 12g/L after supplementation of a constant concentration of 1% 1-propanol in fed-batch mode. A PHBHV copolyester with 8mol.-% 3HV in PHA was obtained. Later, the same group of authors reveled that exposure of C. necator to ethanol or H2O2 at the early stationary phase increases PHB production by about 30%. Mechanistically, the authors assumed that H2O2 enhances the pentose phosphate pathway, which increases the intracellular pool of reduction equivalents (higher ratio of NADPH/NADP+). This slightly inhibits the citric acid cycle (TCA), and shifts the acetyl-CoA flux to PHB biosynthesis and boosts the enzymatic activities of 3-ketothiolase and acetoacetyl-CoA reductase, while PHA synthase activity remains unaffected. The beneficial role of ethanol addition was also explained by the increased pool of reduction equivalents NAD(P)H, which occur due to the ethanol oxidation catalyzed by alcohol dehydrogenase. In addition to PHA overproduction provoked by these stressors, molecular mass was significantly higher under stress conditions than in control cultivation. In particular, molecular mass values were dependent on stress factor concentrations.118 Application of exogenous stress, provoked by oxidants and/or solvents thus displays a simple approach to significantly boost PHB biosynthesis in C. necator.
Only recently, this group revealed that 3HB protects the enzyme lipase not only against heat-mediated denaturation but also against oxidative damage if exposed to metal ions [Cu2+] and H2O2; its chaperon effect was higher than that of trehalose and similar to that of hydroxyectoine. Considering the fact that the PHB-producing C. necator H16 displays 16.5-fold higher intracellular 3HB levels than it´s PHB non-producing mutant C. necator PHB-4, the cyclic PHB metabolism might maintain a higher intracellular 3HB level which improves stress resistance of bacteria capable of parallel PHB synthesis and degradation. As a potential technological application, the authors suggest the use of 3HB as an efficient stabilizer in enzyme formulations.97
C.necator´s tolerance to elevated Cu2+ concentrations, by the way, gave rise to the name of this metalophile bacterium; “Cupriavidus necator” is the Latin expression for a “killer enduring high copper concentrations”.119 This initiated the search for additional metalophile PHA producers. In this context, Chien et al.120 found further evidence that the tolerance of microbes to heavy metals might be linked to its PHB synthesis. Cupriavidus taiwanensis EJ02, a strain phylogenetically related to C. necator, was investigated in their study in the presence of Cd2+ ions. C. taiwanensis EJ02 tolerated up to 5mM Cd2+ ions when grown in complex media, which is about the five-fold concentration compared to PHA-negative C. taiwanensis strains. Resistance towards Cd2+ ions by C. taiwanensis EJ02 was further increased up to 7mM after supplementing defined carbon sources, which boost PHB biosynthesis in this organism. These outcomes go in parallel to studies revealing the high potential of diverse microalgal strains, which can efficiently be cultured in environments highly polluted by heavy metals like Cd2+, Pb2+, or even hexavalent Cr, or other severe toxins like formaldehyde. Analogous to increased PHA biopolyester synthesis by C. taiwanensis, such microalgae generate diverse valued products like pigments or valued lipids under these environmentally stressful conditions reviewed by Koller M et al.121 and Koller M et al.122
The study presents a number of promising outcomes from novel PHA production process based on a number of phylogenetically highly variable extremophiles. Some of these concepts were already tested under controlled conditions in laboratory bioreactors of different size and operational regimes, ranging from simple shaking flask setups, discontinuous bioreactor processes in batch- and fedbatch mode, until more sophisticated solutions like cyclic batch, cyclic fedbatch and continuous chemostat processes. Many of these processes delivered enough polymeric products to demonstrate that the quality of PHA from extremophiles is at least competitive with PHA from well-known mesophilic production strains. Mainly those presented extremophile processes which are based on the combination of robust, extremophile strains, abundantly available feedstocks, and continuous operation mode hold promise for a high-throughput industrial-scale application in a not too distant future. Considering the fact that the research community already has more or less clear ideas about feasible inexpensive feedstocks for “White Biotechnology”, such as for PHA-production, further process optimization has to focus on higher energy efficiency of the processes. In this context, the application of extremophiles provides the change to safe costs for sterility precautions, cooling, or heating.
Nevertheless, the route in the direction of routinely implementing extremophile PHA producers is still cumbersome. What is needed are further screening studies to explore novel, even more powerful extremophile production strains which display growth and PHA accumulation kinetics comparable to the performance of mesophilic strains, and, at the same time, are well adapted to the conversion of inexpensive raw materials without the need for excessive upstream procedures. In addition to the wealth of wild type strains provided by nature, genetic engineering might be reasonable, as the case arises, to fine tune selected production strains. This encompasses the disclosure of metabolic bottle necks to enhance the conversion of defined feedstocks, which, for example, could be a viable tool to facilitate the conversion of galactose from hydrolyzed whey by Hfx. mediaterranei. Successful attempts in the direction of enhanced substrate conversion by halophiles were presented in this review for the halophile Halomonas sp. The knockout of genes responsible for by-product formation, as successfully demonstrated by the development of EPS-negative mutants of haloarchaeal PHA producers, would contribute to achieve higher product yields. Further, deletion of depolymerase genes offers a chance to further improve process productivity by impeding the intracellular product degradation in late stages of the process, as evidenced in the case of thermophilic PHA production by Chelatococcus sp., or as reported for halophile Halomonas sp.
As the downside of the medal, extremophile cultivation conditions request special requirements to the bioreactor equipment and its surroundings, such as improved reactor materials, resistant sensors, and smart sealing systems. This opens the door for new engineering developments, as well as investigations to improve materials and electronic devices.
Despite all these challenges, data from bench-scale experiments and current R&D efforts on pilot scale give rise to optimism that the application of extremophiles for production of PHA and other marketable bioproducts by no way constitutes an academic shenanigan, but is among the top-objectives towards an efficient and sustainable bio-based industry of the future. Table 1 summarizes the most promising outcomes discussed in this review, collecting the production strains, the extremophile conditions and carbon sources applied, the type of PHA produced, productivity and production scale.
Production strain |
Extremophile conditions and carbon source |
Type of PHA produced |
Production scale and PHA Productivity |
Ref. |
Hfx. Mediterranei(Archaea) |
25 % marine salts [halophile] Starch (20g/L) |
PHBHV [in original literature: “PHB”] |
Stable continzuous cultivation over 3 months in 1.5 L bioreactor; T = 38 °C 6.5 g/L PHA on starch |
|
Hfx. Mediterranei[Archaea] |
15 % NaCl [halophile]; |
PHBHV |
Fedbatch fermentation in 10 L bioreactor; 0.21 g/(L•h) |
|
Hfx. Mediterranei |
20 % NaCl [halophile], Hydrolyzed whey permeate Hydrolyzed whey permeate plus GBL |
PHBHV P(3HB-co-3HV-co-4HB) |
42 L bioreactor; 0.09 g/[L·h], 12.2 g/L PHBHV] 0.14 g/[L·h], 14.7 g/L [3HB-co-3HV-co-4HB] |
|
Hfx. Mediterranei[Archaea] |
15 %, [halophile]; Crude glycerol phase; Crude glycerol phase plus GBL |
PHBHV P[3HB-co-3HV-co-4HB] |
42 L / 10 L bioreactor; 0.12 g/[L·h], 16.2 g/L PHA 0.10 g/[L·h], 11.1 g/L PHA |
|
Hfx. Mediterranei[Archaea] |
23.4 % NaCl [halophile]; Extruded rice bran plus extruded corn starch |
PHBHV |
pH-stat feeding strategy in 5 L bioreactor; 24.2 g/L PHA |
|
Hfx. Mediterranei[Archaea] |
23.4 % NaCl [halophile]; Extruded corn starch / yeast extract |
PHBHV |
pH-stat feeding strategy in 5 L bioreactor; |
|
Natrinema pallidum JCM 8980 [Archaea] |
25 % NaCl [halophile]; Starch |
PHBHV |
Shaking flak scale; PHA content in CDM of about 53.14 wt.-% |
|
Halomonas campaniensis LS21 [Bacteria; Gram-negative] |
27 g/L NaCl [halophile] and pH-value 10 [alkaliphil]; alkaline seawater and artificial carbonaceous kitchen waste |
PHB |
Open cultivation for 65 days 26 % PHA in CDM [wild type strain], 70 % PHA in CDM [engineered strain] |
|
Halomonas TD01 [Bacteria; Gram-negative] |
60 g/L NaCl [halophile]; glucose |
PHB |
Open continuous cultivation process for 56 h; 64 g/L PHA |
|
Halomonas TD01 [genetically engineered] [Bacteria] |
60 g/L NaCl [halophile]; Glucose, propionic acid |
PHB, PHBHV |
Open continuous cultivation process for 56 h; 80 g/L PHB, 56 g/L PHBHV |
|
Bacillus megaterium uyuni S29 [Bacteria; Gram-positive] |
45 g/L NaCl [halophile]; glucose |
PHB |
Shaking flak scale; 2.2 g/L PHB, 0.10 g/[L·h] |
|
Pseudomonas extremaustralis [Bacteria; Gram-negative] |
28 °C, but growth also at lower temperature [cryophile] Contaminated soil samples |
PHB |
Static cultivation on poly[styrene] biofilms biofilm vs. shaking flask cultures PHA productivity not reported |
|
Thermus thermophilus [Bacteria; Gram-negative] |
75 °C [thermophile]; Gluonate Octanoate |
scl-co-mcl-PHA mcl-co-scl-PHA |
Shaking flask scale;Up to 40 % PHA in CDM |
|
Synechococcus sp. MA19 [Cyanobacteria; Gram-negative] |
50 °C 2 % CO2 |
PHB |
Photoautotrophic cultivation in simple bottles; 20- 27 % PHA in CDM |
|
Spirulina sp. MA19 [Cyanobacteria; Gram-negative] |
50 °C [thermophile] CO2 |
PHB |
Autotrophic cultivation2.4 g/L PHB |
|
Chelatococcus sp. strain MW10[Bacteria; Gram-negative] |
55 °C [thermophile]Glucose |
PHB |
2 L fedbatch fermentation [ca. 3 g/L PHB];42 L cyclic batch fermentation[ca. 4 g/L PHB];42 g/L cyclic fed-batch fermentation [ca. 16.8 g/L PHB] |
|
Chelatococcus daeguensis TAD1[Bacteria; Gram-negative] |
50 °C [thermophile] Glucose Glycerol |
PHB |
Shaking flask scale 3.44 g/L PHB 80 % PHB in CDM |
|
Chelatococcus daeguensis TAD1[Bacteria; Gram-negative] |
50 °C [thermophile]Glycerol plus cocktail of nitrogen sources |
PHB |
Two-stage fedbatch process 17.4 g/L PHB, 0.434 g/[L·h] |
|
Bacillus shackletonii K5[Bacteria; Gram-positive] |
45 °C Glucose |
PHB |
Batch mode cultivation 7 g/L PHB |
|
Table 1 Overview on most promising PHA production processes by extremophiles
Table Abbreviations: CDM: Cell dry mass; GBL: γ-Butyrolactone; Hfx: Haloferax; mcl: medium chain length; PHA: Polyhydroxyalkanoate; PHB: Poly (3-hydroxybutyrate); scl: short chain length
None.
The author declares no conflict of interest.

©2017 Koller. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.