Journal of
eISSN: 2378-3184


Research Article Volume 12 Issue 3
South Dakota Department of Game, Fish and Parks, McNenny State Fish Hatchery, USA
Correspondence: Michael E. Barnes, South Dakota Department of Game, Fish and Parks, McNenny State Fish Hatchery, 19619 Trout Loop Spearfish, South Dakota, USA
Received: November 27, 2023 | Published: December 13, 2023
Citation: Gerber AL, Voorhees JM, Huysman N, et al. Stress response of juvenile rainbow trout reared at three densities. J Aquac Mar Biol. 2023;12(3):292-295. DOI: 10.15406/jamb.2023.12.00386
High rearing densities (kg of fish per rearing unit volume) can impact fish health and growth. This study evaluated the stress levels (as indicated by blood glucose) and growth of juvenile Shasta strain rainbow trout (Oncorhynchus mykiss) reared at three densities in 1.8-m diameter circular tanks for 111 days. Initial and final rearing densities for each treatment were: low (4.48 and 47.56 kg/m3), medium (5.96 and 60.60 kg/m3), and high (7.49 and 72.42 kg/m3). Throughout the experiment, blood glucose levels from individual fish were not significantly different among the three density treatments. At the end of the experiment, total tank weights and gain were significantly greater in the high-density treatment than the medium-density treatment, which in turn was significantly greater than the low-density treatment. However, percent gain was significantly greater in the low-density treatment. Feed conversion ratio was significantly lower in the high-density treatment compared to the low-density treatment, with the ratio in the medium-density treatment similar to the other two treatments. The results of this study indicate that higher rearing densities do not negatively impact juvenile Shasta strain rainbow trout stress or hatchery rearing performance.
Keywords: rainbow trout, Oncorhynchus mykiss, density, stress, circular tank
The density of fish (kg per rearing unit volume) varies during hatchery rearing.1 While fish production is maximized at high rearing densities, growth is negatively impacted if rearing tank carrying capacity is exceeded.2,3 High rearing densities can also affect the health and physiological functions of fish.4,5 Numerous studies have reported poorer water quality at higher rearing densities, along with decreased fish growth, impaired fin condition, and lower feed conversion ratios.5–10 Higher rearing densities can also negatively impact the post-stocking survival of hatchery-reared fish.1 ,11–15
The effects of rearing density and stress in fish have not been well studied. In general, physiological stress responses in fish increase during periods of intense crowding during netting and routine fish culture.16–19 The two studies specifically examining stress and rearing density have produced different results. Iguchi et al.,9 reported increasing cortisol levels in ayu (Plecoglossus altivelis) with increasing rearing densities. Contrarily, Freestone et al.4 did not observe any effect on glucose as a measure of stress in a short-term study with rainbow trout (Oncorhynchus mykiss). However, the Freestone et al.,4 study lasted only 14 days, only evaluated two different densities, and also incorporated feeding and starvation treatments.
The longer-term effects of high rearing densities on rainbow trout have not been evaluated. It is possible that physiological stress is contributing to the impaired fish health, decreased post-stocking survival into natural environments, and undesirable behaviors observed in fish reared for long periods of time at higher densities.5,6,8–10,14,15 Thus, the objective of this study was to document the stress response of juvenile rainbow trout, as indicated by blood glucose levels, subjected to long-term changes in rearing density.
This experiment was conducted at McNenny State Fish Hatchery, rural Spearfish, South Dakota, USA over a 111-day period between 25 May 2021 and 14 September 2021. De-gassed and aerated well water (11 °C; total hardness 360 mg/L CaCO3; alkalinity as CaCO3, 210 mg/L; pH 7.6, total dissolved solids 390 mg/L) was used throughout the experiment. Eighteen, 1.8-m diameter, fiberglass circular tanks contained juvenile Shasta strain rainbow trout (Oncorhynchus mykiss) (mean±SE; initial weight: 6±0 g; initial length: 82±1 mm) at three different densities (n=6). Initial densities in the low, medium, and high-density tanks were 4.48 kg/m3 (9.1 kg/tank, approximately 1,500 fish), 5.96 kg/m3 (12.1 kg/tank, approximately 2,000 fish), and 7.49 kg/m3 (15.2 kg/tank, approximately 2,500 fish), respectively. All tanks were near-fully covered20 with four aluminum angles (each side 2.5-cm wide x 57.15-cm long) vertically-suspended for environmental enrichment21 (Figure 1).
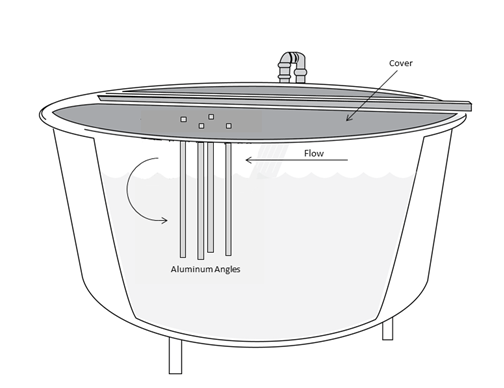
Figure 1 Schematic of a covered 1.8-m diameter circular tank with a suspended array of four aluminum angles for environmental enrichment, with the peak of the angle facing in the direction of the water flow.
Feed amounts were calculated using the hatchery constant method22 with a projected feed conversion rate of 1.1. Tanks were projected at a growth rate of 0.07 cm/day from May 25 to June 15, and then all growth rates were increased to 0.075 cm/day for the remainder of the study. Total feed amounts per tank were 76.06 kg, 101.32 kg, and 126.73 kg, for the low, medium, and high-density treatments, respectively. Fish were fed 1.5 mm, extruded floating feed (Protec, Skretting USA, Tooele, Utah, USA) every 15 minutes during daylight hours using automatic feeders. Moribund fish were removed, counted, and recorded weekly.
At approximately three-week intervals and at the end of the experiment, three fish from each tank were randomly removed from each tank and euthanized using a lethal dose of 200 mg/L of tricaine methane sulfonate (MS-222; Tricaine-S, Syndel, Ferndale, WA, USA). They were then immediately measured (total length) to the nearest mm, weighed to the nearest g, and n blood was collected via caudal fin severance. Glucose (mg/dL) measurements were obtained using a blood glucose monitor (AccuCheck Aviva Plus; Roche Diabetic Care, Indianapolis, Indiana, USA).
At the end of the experiment all the fish in each tank were weighed to the nearest 0.1 kg. The following equations were used:
Data were analyzed using the statistical program SPSS (24.0; IBM; Armonk, New York, USA) with significance predetermined at p <0.05. Percentage data were log transformed prior to analysis.23 If one-way analysis of variance (ANOVA) indicated significant differences among the treatments, Tukey’s post-hoc means testing procedure was conducted. Because the tanks (and not individual fish) were the experimental unit, individual fish data (length, weight, glucose) were first averaged by tank, and the averages used for subsequent analysis.
Blood glucose was not significantly different among the rainbow trout reared at the three density treatments (Table 1). Final tank weight, gain, percent gain, and feed conversion ratio were all significantly different among the treatments (Table 2). Final tank weights and gain were significantly greater in the high-density treatment than the medium-density treatment, which in turn was significantly greater than the low-density treatment. However, percent gain was significantly greater in the low-density treatment. Feed conversion ratio was significantly lower in the high-density treatment compared to the low-density treatment, with the ratio in the medium-density treatment similar to the other two treatments.
|
|
Density |
p |
||||||||
|
|
Low |
Medium |
High |
|||||||
|
Day 0 (initial) |
86 |
± |
9 |
86 |
± |
9 |
86 |
± |
9 |
|
|
Day 26 |
103 |
± |
6 |
102 |
± |
5 |
105 |
± |
8 |
0.950 |
|
Day 52 |
113 |
± |
8 |
103 |
± |
5 |
115 |
± |
4 |
0.316 |
|
Day 63 |
72 |
± |
4 |
96 |
± |
8 |
80 |
± |
6 |
0.054 |
|
Day 83 |
90 |
± |
34 |
85 |
± |
4 |
90 |
± |
5 |
0.616 |
|
Day 111 (Final) |
72 |
± |
4 |
70 |
± |
3 |
72 |
± |
6 |
0.973 |
|
Overall |
90 |
± |
4 |
92 |
± |
4 |
91 |
± |
3 |
|
Table 1 Mean (±SD) blood glucose (mg/dL) levels from rainbow trout reared at one of three densities in 1.8-m diameter circular tanks (n=6)
|
|
Density |
p |
||||||||
|
|
Low |
Medium |
High |
|||||||
|
Initial weight (kg) |
9.1 |
± |
0.0 x |
12.1 |
± |
0.0 y |
15.2 |
± |
0.0 z |
0.001 |
|
Final weight (kg) |
96.6 |
± |
2.1 x |
123.1 |
± |
2.0 y |
147.1 |
± |
2.1 z |
0.001 |
|
Gain (kg)1 |
87.5 |
± |
2.1 x |
111 |
± |
2.0 y |
132 |
± |
2.1 z |
0.001 |
|
Gain (%)2 |
96.1 |
± |
2.3 z |
91.7 |
± |
1.6 zy |
86.7 |
± |
1.4 y |
0.009 |
|
FCR3 |
1.15 |
± |
0.03 y |
1.10 |
± |
0.02 zy |
1.04 |
± |
0.02 z |
0.010 |
Table 2 Mean (±SD) weights, gain, percent gain, and feed conversion ratio (FCR) of tanks of rainbow trout reared at one of three densities. Means in a row followed by different letters are significantly different (n=6)
1Gain (kg) = final tank weight-initial tank weight
2Gain (%) = 100 x (gain/initial tank weight)
3Feed conversion ratio (FCR) = food fed/gain
At the end of the experiment, no significant differences were observed in individual fish length, weight, specific growth rate, or condition factor among the treatments (Table 3). Mortality was negligible at less than 0.1% in all of the tanks. Mean final densities for the low, medium, and high-density treatments were 47.56, 60.60, and 72.42 kg/m3.
|
|
Density |
|
||||||||
|
|
Low |
Medium |
High |
p-value |
||||||
|
Length (mm) |
176 |
± |
3 |
170 |
± |
7 |
165 |
± |
4 |
0.305 |
|
Weight (g) |
63 |
± |
5 |
57 |
± |
7 |
49 |
± |
4 |
0.226 |
|
SGR1 |
2.10 |
± |
0.07 |
1.97 |
± |
0.13 |
1.87 |
± |
0.07 |
0.262 |
|
K2 |
1.16 |
± |
0.04 |
1.12 |
± |
0.04 |
1.09 |
± |
0.02 |
0.403 |
Table 3 Mean (±SD) length, weight, specific growth rate, and condition factor from individual rainbow trout reared in 1.8-m diameter circular tanks at one of three densities (n=6)
1Specific growth rate (SGR) = 100 × [ln (end weight) – ln (start weight)]/ (number of days)
2Condition factor (K) = 105 × (weight /length3)
The results of this study indicate that for relatively-domesticated rainbow trout, such as the Shasta strain,24,25 reared at high densities, stress is not an issue. Blood glucose, which typically increases following a stressor,26 did not differ among any of the three density levels. In addition, gain, specific growth rate, feed conversion ratio, and other indirect measures of physiological stress also were unaffected by density. These results are supported by the observations of Freestone et al.,4 who also noted the lack of density effects on blood glucose in rainbow trout subjected to one of two densities for a very short duration. Papoutsoglou et al.27 also reported no density-dependent effects on blood glucose. In contrast, Leatherland and Cho,28 Trenzado et al.,29 Yarahmadi et al.,30 and Wydoski31 all observed a positive relationship between blood glucose and fish rearing density. Iguchi et al.9 also found that increased densities during rearing of ayu (Plecoglssus altivelis), a non-salmonoid fish, caused elevated stress responses. Contrarily, Vijayan and Leatherland32 reported that blood glucose decreased with increasing rearing densities. The differing results among these studies are likely explained by the differences in fish species, genetic strains, sizes, experimental densities used, water chemistry, and rearing histories.
The positive relationship between densities and both gain and final tank weights was expected. Tanks containing more fish and being fed at the same rate as tanks containing fewer fish will obviously gain and weigh more at the end of a rearing period if carrying capacities are not exceeded.2 The significant improvement in percent gain at the lowest density was also expected. Improved growth at lower densities has been well-documented in numerous studies.6,10,33,34 However, the relationship between feed conversion ratio and density observed in this study was surprising. Increased feed conversion ratios are typically observed at higher, in relation to lower, rearing densities. This has been reported extensively in salmonids.2,7,11,15,33,35,36 Two studies with salmonids reported no relationship between feed conversion ratio and rearing density.10,37 This is the first study to document an improvement in feed conversion ratio as rearing density increased. This could possibly be explained by the densities and feeding rate used in this study, particularly if the lower densities tanks of fish were slightly overfed. The feed conversion ratios observed in this study are similar to those reported by Voorhees et al.38,39 for rainbow trout reared at similar densities.
Individual fish lengths, weights, and specific growth rates at the end of this study were not significantly different among the density treatments. This is likely because of the small sample sizes resulting from tanks as the experimental unit.40 While the mean values for all of these individual fish variables follow a pattern of increasing with decreasing densities, as was expected, none of these values are statistically significantly different. The specific growth rates observed in this study were similar to those reported previously for Shasta strain rainbow trout.41
The densities used in this study are similar to those typically used for growing rainbow trout and other salmonids at production‑scale.38,42,43 Based off of the results of this study, relatively high densities can continue to be used with minimal impacts on fish stress, growth, or feed utilization efficiencies. Future research could focus on the effects of densities even higher or lower than those used in this study.
Thanks to Jade Freestone for her assistance with this study.
The authors declare that there are no conflicts of interest.

©2023 Gerber, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.