Journal of
eISSN: 2572-8466


Research Article Volume 10 Issue 5
Department of Bioengineering, University of California, USA
Correspondence: Bill Tawil, Department of Bioengineering, University of California, Los Angeles, USA
Received: October 01, 2023 | Published: October 17, 2023
Citation: Steward N, Tawil B. Liver cirrhosis: physiology, pathology, market analysis, treatments. J Appl Biotechnol Bioeng. 2023;10(5):149-157. DOI: 10.15406/jabb.2023.10.00342
Azadirachta indica A. Juss is known as the tree of life for its diverse pharmacological applications. This study evaluated the effect of the anxiolytic type of bark of A. indica and mechanisms of action on adult zebrafish. Initially, EtCNeem toxicity was detected against Artemia salina, eliminated with the saponification reaction. The antioxidant potential of the fractions was evaluated. The fraction with higher antioxidant potential was submitted to the preliminary chemical prospecting, FT-IR, and the phenol and flavonoid content was determined. The animals were treated with the most promising antioxidant fraction (1.0; 2.5 or 5.0 mg/mL, i.p.) and underwent acute toxicity, open field tests and Light & Dark Test, evaluating the participation of serotonergic and GABAergic systems in the anxiolytic test. As a result, we selected the fraction of ethyl acetate (F-EtOAc) rich in phenolic and flavonoid compounds with antioxidant potential, which had a sedative and anxiolytic effect on adult zebrafish through serotonergic and GABAergic systems. F-EtOAc prevented alcohol-induced chronic anxiety in adult zebrafish. The results suggest that F-EtOAc is an anxiolytic agent mediated via the GABAergic and serotonergic system.
Keywords: neem barks, acute and chronic anxiety, adult zebrafish, GABAA, 5-HT
Liver cirrhosis has demonstrated a rise in mortality and morbidity in underdeveloped and developing countries.1 Rather than being categorized as a disease, cirrhosis has been broken down into specific clinical stages based on the state of the liver, with mortality percentages ranging depending on the stage.6 Patients diagnosed with cirrhosis will eventually approach death, with liver transplantation being the only “treatment” known to avoid this fate.6 The market demand for liver treatment devices, preventative strategies, and transplantation alternatives has continuously increased due to the shortage of liver donors and vast donor waitlist.7,8 Moreover, the rising prevalence of biological devices and optimized materials has fueled the industry shift to a growing field for cirrhosis treatment–tissue engineering.9 Herein, we review liver physiology and the process of cirrhosis onset, the various types and specificities of extracorporeal liver devices, and cirrhosis market analysis including, market size, distribution, and trends. We close by reviewing emerging tissue engineering applications that hope to bridge liver transplantation.
Healthy tissue
The liver plays an important role in all bodily functions and interacts with almost every organ in the body.10 It is the largest organ in the body, accounting for 2% of a person’s weight. Its main functions include digestion, filtration, metabolism and detoxification, protein synthesis, and storage of vitamins and minerals. Specifically, digestion and metabolism are carried out by the liver as it interacts with both the endocrine system and gastrointestinal system. It also stores iron and copper which perform cholesterol homeostasis.10 The liver has two supplies of blood coming from the portal vein and the hepatic artery and contains about a pint of blood at any moment.10,11 In one day the liver filters around 250 gallons of blood, which is more than a liter per minute.11
The liver is made up of small units called lobules, each of which contains sets of portal veins, hepatic arteries, and bile ducts (e.g. the portal triad).12. They are made up of cells called hepatocytes which have different roles based on their location in the lobule. Around the portal vein, hepatocytes function the most efficiently and regenerate first because they are close to the blood and nutrients. This region’s main function is oxidative metabolism. Hepatocytes that are farthest away from the portal triad have the lowest perfusion and play an important role in the detoxification of drugs and other molecules. Hepatocytes also create tubes that collect bile and facilitate the flow of bile out of the liver. Blood and bile flow in opposite directions with blood entering the liver and bile leaving the liver. The space of Disse is the area between the sinusoid and hepatocytes that contains various proteins.12 The hepatocytes contain microvilli that extend into this space to allow for proteins and nutrients from the blood and in the space to enter them. This space also contains macrophages, to clear out pathogens, and stellate cells, to store fat.12
The main liver functions, as stated above, are bile production, vitamin storage/metabolism, drug metabolism, and bilirubin metabolism. All material that is not filtered out in the kidney is done by the liver in a fluid called bile.11,12 It breaks down lipids by secreting salts and acids and is produced by hepatocytes. Vitamins that are fat-soluble are absorbed by the intestines and transported to the liver, where there are stored and/or metabolized. To facilitate drug metabolism, the liver uses two routes: lysosomes and biotransformation. Biotransformation is a two-step process that breaks down drugs into a hydrophilic form so they can be secreted into the blood or bile. The last main function of the liver is heme breakdown. After heme is broken down into bilirubin, the liver receives it from circulation and is processed to a hydrophilic state. Most of this bilirubin is excreted in the bile. Lastly, the liver plays a role in hormone function and facilitates plasma protein synthesis.12
Diseases
Cirrhosis is the end stage of liver disease and can result from viral infections, toxins, autoimmune disease, or hereditary issues that cause liver injury.11,12 Some of the main causes include alcohol abuse and chronic hepatitis infection. The onset of cirrhosis is due to fibrosis in the liver which is caused by the secretion of the TGF-β growth factor from stellate cells in the space of Disse. Cirrhosis is a secondary disease that follows a chronic injury that forms scar tissue over time. The onset of the disease can be separated into 3 stages: healthy liver, chronic liver injury, and cirrhosis.6,12 The liver cannot function at all if it reaches this end state, which limits protein production and substance detoxification. These issues can lead to portal hypertension which induces varice and hemorrhoid formation, coagulopathy, and hyperestrinism. In addition, patients with cirrhosis can develop jaundice because of lowered bilirubin metabolism.11,12
When cirrhosis occurs, multiple cells are damaged, including hepatocytes, stellate cells, macrophages, and endothelial cells. Stellate cells are able to transform into myofibroblasts and secrete collagen after exposure to cytokines, which leads to fibrosis. Endothelial cells form a lining that contains capillaries to allow the exchange of nutrients and proteins to the hepatocytes. When cirrhosis occurs, there are fewer of these capillaries, which promotes fibrosis. The macrophages in the space of Disse release factors when exposed to various pathogens that play a role in liver fibrosis. Lastly, hepatocytes, when damaged, release inflammatory factors and reactive oxygen species that can activate the stellate cells and, in turn, liver fibrosis (Figure 1).12
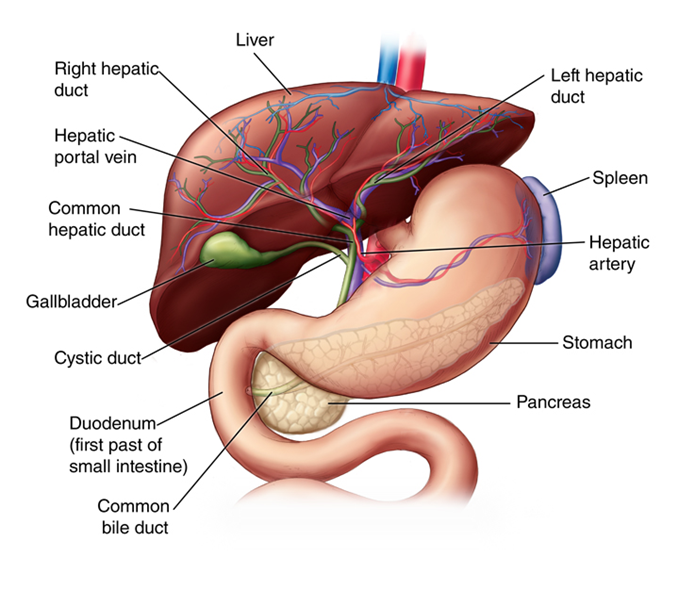
Figure 1 Liver anatomy50 Arrangement of vessels and different organ systems connected to liver.
Liver damage can be caused by unhealthy habits, prior disease, and viral infection. Acute hepatitis is a sign of Hepatitis A and E which can cause acute liver damage. While Hepatitis B, C, and D can also cause acute hepatitis, they result in chronic infection. Primary biliary cholangitis (PBC) is an autoimmune disease that chronically affects the liver, leading to cirrhosis. Alcohol abuse also has severe effects on the liver since the organ is responsible for breaking down alcohol.11,12 Constant consumption of alcohol damages cells due to the buildup of txic metabolites, leading to cirrhosis. All of these issues that lead to cirrhosis can eventually result in malignancy of the liver, such as hepatocellular carcinoma. Non-alcoholic fatter liver disease (NAFLD) can range in its effects on the liver, with the most serious being cirrhosis.12 NAFLD can become a chronic disease that will eventually require a liver transplant (Figure 2).
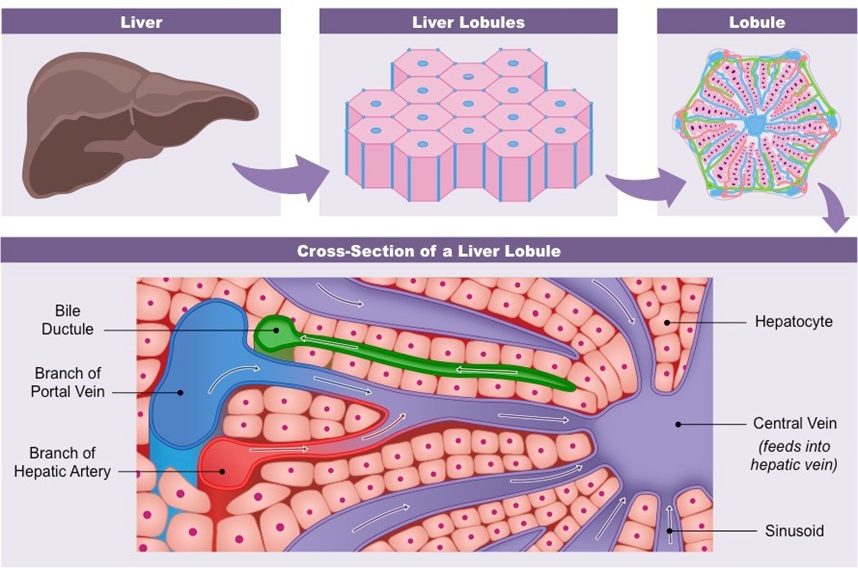
Figure 2 Specific lobule structures that make up the liver.51
Market size
In the US, the 12th most common causes of death are chronic liver disease and cirrhosis together.13 According to the CDC, 34,174 deaths were associated with liver cirrhosis in 2016.1 The increasing numbers of those with alcoholic and non-alcoholic fatty liver diseases will contribute to this number being tripled by 2030.1,14 In addition, 570,730 patients above the age of 18 were hospitalized in 2014 which was over a 30% increase in patient admissions for cirrhosis in only 6 years.15 The fibrotic progression of these diseases to cirrhosis is associated with increasing type 2 diabetes mellitus, obesity, and age, which attributes to the shifting age demographic.14,16 There have been several studies focusing on the United States that indicated a yearly healthcare expenditure for liver cirrhosis to be around $2.5 billion, with indirect costs going up to $10.6 billion each year.4,15 This number has surely increased seeing as these estimates were from 2004 and trends have shown the prevalence of chronic liver disease and cirrhosis has shown an upward trend (Figure 3).15
Cirrhosis is among the leading causes of morbidity and mortality in more developed countries.1 Cirrhosis of the liver was the cause of 1,000,000 deaths in 2010, which was 33% more than in 1990.17 This is seen in Figure 4. This is due to the rise in hepatitis B, hepatitis C, and alcohol. It is ranked 14th as the leading cause of death globally in people above the age of 18,17 resulting in 170,000 deaths per year in Europe,18 with Egypt, Moldova, and Mongolia having some of the greatest cirrhosis mortality rates in the world as seen in Figure 4B.19 Hepatitis infection, alcohol abuse, and general lack of health upkeep are seen as the most common causes in more developed countries. In Africa and Asia, on the other hand, hepatitis B infection is seen as the most common cause. Because the disorder is undiagnosed, the prevalence of cirrhosis is probably higher than reported.19
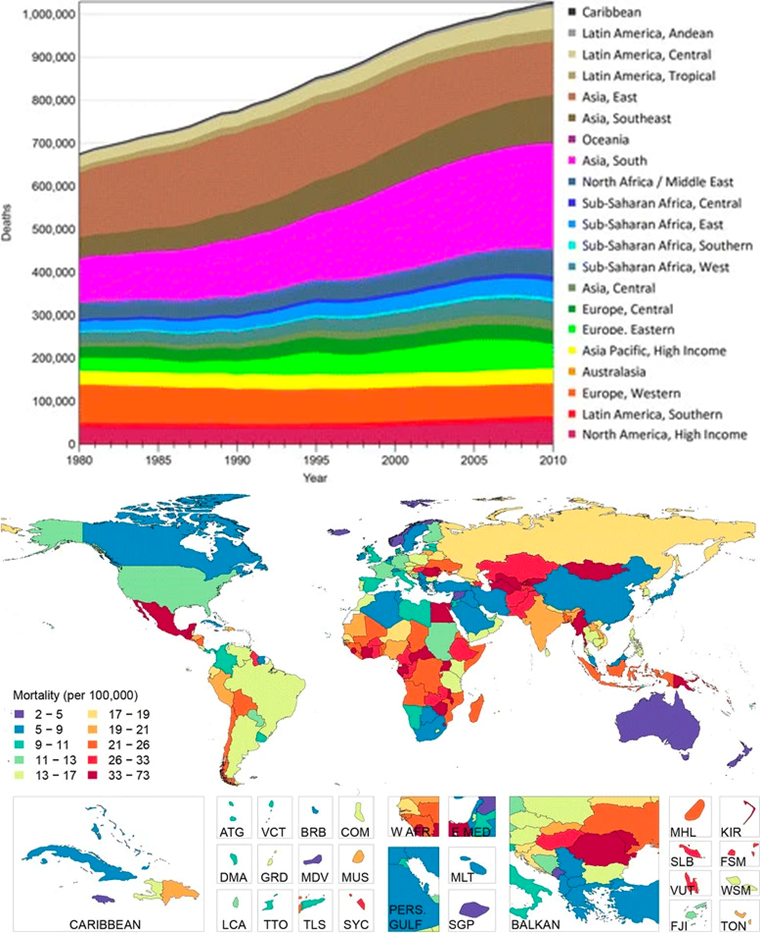
Figure 4 Worldwide statistics for liver-cirrhosis-associated deaths24 (A) Number of liver cirrhosis deaths from 1980 to 2010 globally. (B) Global distribution of age-adjusted liver cirrhosis mortality (per 100,000) in 2010.
Liver cirrhosis is most common in people between the age of 45 and 64, with studies ranking it as the fourth leading cause of death for that age range (Figure 5). When combined with liver cancer, these two diseases account for 3.5% of deaths globally.20 The number of deaths reported among 45- to 64-year-olds increased from 16,746 in 198013 to 22,102 in 2018.21 From 1979 to 2008, the age at death decreased in people below 45 years by 14%, and deaths among people older than 75 years increased by 16%.13 While the incidence of cirrhosis in individuals between 25-44 is much lower compared to older age groups, a study showed the greatest change in yearly mortality for cirrhosis was in people aged 25-34; 10.5% during 2009-16, seen in Figure 5.1 This could be related to similar trends seen in alcohol use disorder and all alcohol-related liver disease deaths.1
Doctors approach cirrhosis treatment by treating the primary disease since some of the diseases that cause cirrhosis can be cured.3 Many treatments include taking medicines and making lifestyle changes to help prevent further complications, but the only true “cure” for the disease is liver transplantation.3,4 Every year, roughly 25,000 liver transplants are performed with about a 90% 1-year survival rate, and about 8000-9000 in the U.S. with an 86% survival rate at 1 year.8,11 As seen in Figure 6A, the number of liver transplants being performed has steadily increased with higher rates after the COVID-19 pandemic.2 Mortality rates for patients among adult liver transplant recipients range between 5-20% depending on the procedure and patient demographics.2 With the success of liver transplantation comes the issue of a donor shortage in both children due to the scarcity of matched pediatric donors and in adults, because of the growing number of patients being added to the waitlist.8 In 2020, 24936 candidates were on the waitlist for liver transplant.2 In 2017, of the adults listed for a transplant, 58.0% received a transplant, 10.1% died, 23.4% were removed from the without receiving a transplant, and 5.8% were still waiting, as seen if Figure 6B.2
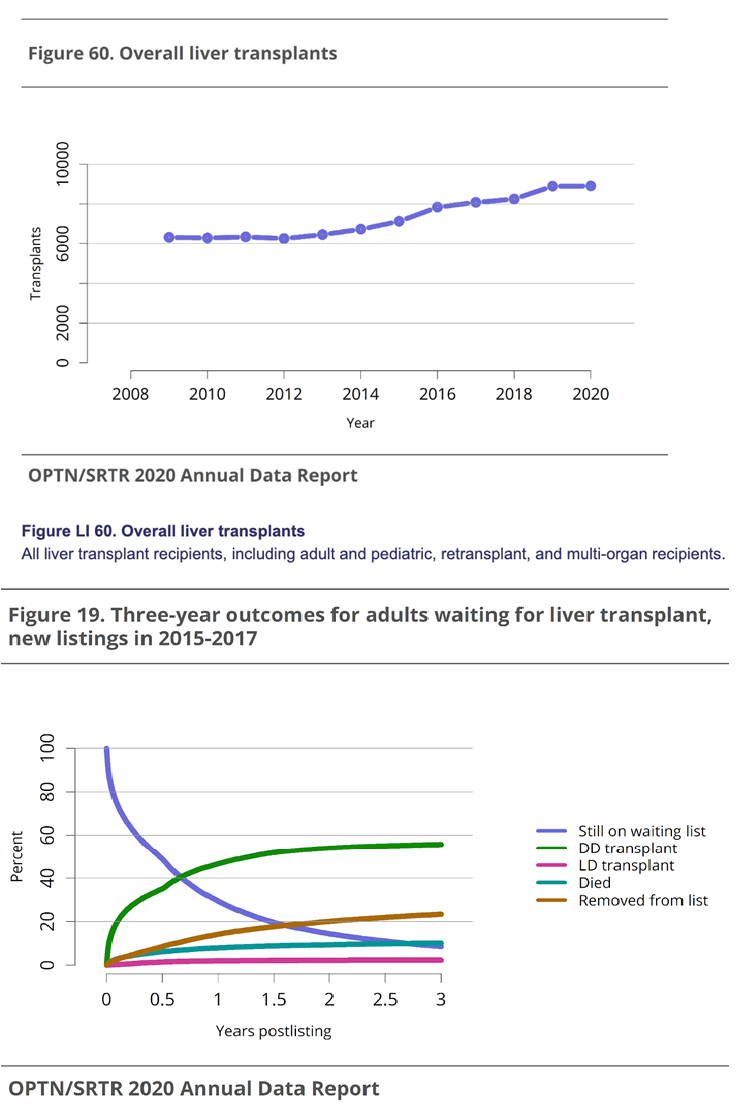
Figure 6 Liver transplant trends in the US30 (A) All liver transplant recipients, for all age groups. (B) Outcomes for adults on the transplant waitlist from 2015-2017.
The increase in patients with cirrhosis/end-stage liver disease has pushed for companies to create alternative tissue engineering and drug approaches. In 2020, the United States cirrhosis treatment market had a $6.29 billion value and is expected to rise to a revenue of $14.86 billion in 2028, as seen in Figure 7.7 Key playmakers who are focusing on the commercialization and regulation of liver restorative treatments are Pfizer Inc., Johnson & Johnson Services, Inc., F. Hoffmann-La Roche Ltd, GlaxoSmithKline plc., Sanofi, Novartis AG, Bayer AG, Bristol Myers Squibb (BMS), Gilead Sciences, Inc., and AbbVie.7

Figure 7 Cirrhosis treatment market across different regions worldwide.20
Market segments and trends
The cirrhosis treatment market can be divided based on the type of treatment, disease type, and region. Different treatments include antiviral drugs, immunosuppressants, vaccines, immunoglobulins, corticosteroids, targeted therapy, and chemotherapy drugs, as seen in Fig. 8A.23 Diseases classified as primary to cirrhosis include, hepatitis, autoimmune diseases, non-alcoholic fatty liver disease, cancer, genetic disorders, etc (Figure 8B).23
Patients with cirrhosis have to be treated for the primary liver disease to lessen the progression of the disease.24 On average, patients are prescribed between 3 and 10 medications, indicating the importance of proper diagnosis and prescription.25 Depending on the type of treatment there are advantages and limitations concerning treating cirrhosis (Table 1). Treatments include immunosuppression for autoimmune hepatitis, venesection for hemochromatosis, and others.24 Patients who have viral hepatitis need antiviral treatments,24 vaccines for hepatitis A and B should also be administered because of decreased antigenic response as cirrhosis progresses.26 Reports indicate hepatitis infection is the highest contributor to cirrhosis development (Figure 9), and treatments for these viral diseases have shown a significant reduction in associated mortality5. A 24% and 66% reduction in mortality was seen in the US and China, respectively, from 1980 to 2010 because of HBA/HBV/HBC prevention efforts.26 Portal hypertension underlies many of the complications of cirrhosis that lead to mortality, which results in the formation of varices and ascites. Treatment includes the use of non-selective beta blockers, which have shown promising results.24
|
Treatment |
Disease |
Advantages |
Disadvantages |
|
Antiviral Drugs |
Hepatitis |
- improve liver fibrosis |
progression of fibrosis not always |
|
Vaccines |
HAV / HBV |
- prevents infection from HAV / HBV |
inadequate access to healthcare and proper |
|
Corticosteroids |
Autoimmune Diseases |
- frequency of cirrhosis decreased in patients |
- strong side effects |
|
Chemotherapy |
Cancer |
- prolongs lifespan of patients with cancer |
- Several chemotherapeutic agents can cause |
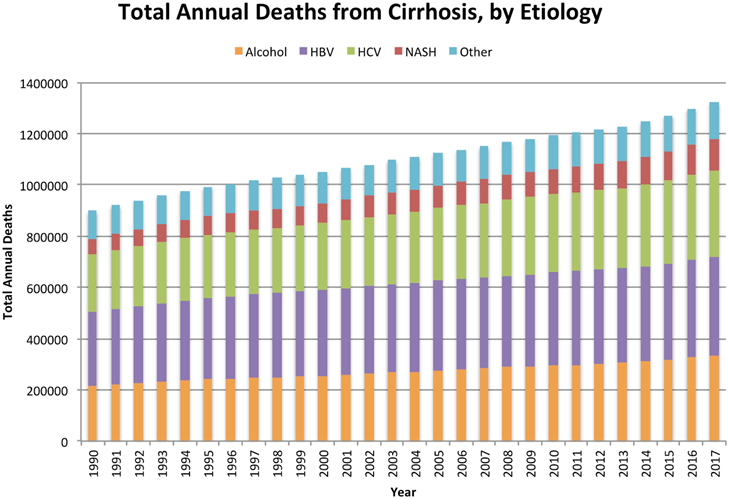
Figure 9 Total annual deaths from cirrhosis by etiology from 1990 to 2017 globally.5
Extracorperal liver devices: artificial vs. bio-artificial devices
The goal of tissue engineering for the liver is the mimic normal liver functions, allowing for recovery and regeneration of the liver.24,27 This strategy can increase the chance of a waitlisted transplant patient receiving a compatible organ or completely prevent the need for a transplant. As explained above, the main reason for liver failure is the accumulation of toxins.5 As so, the main focus of researchers and manufacturer is the detoxification process. Artificial and bio-artificial extracorporeal devices are the main products being studied, both with significant advantages and disadvantages.
Artificial devices
The main principles of artificial liver devices are albumin dialysis, membrane filtration, and the use of adsorbent columns to remove toxins.5 These devices detoxify the blood and plasma using membrane separation techniques associated with columns or suspensions of sorbents (Figure 10).9 Sorbents used include charcoal, anion, or cation-exchange resins.9 Albumin dialysis uses exogenous albumin to remove toxins that are bound from patients’ endogenous albumin.28 Concentration gradient principles are utilized to allow the movement of toxins from the internal albumin to the dialysate.28 This lowers the resistance of protein-bound substances to mass transfer. Another approach is to filter and detoxify the patient’s albumin from the bloodstream using resin binders. The molecular adsorbent recirculating system is the most used liver support system and it uses dialysis, filtration, and adsorption. The three-part system circulated blood a hemodialyzer, then human serum albumin through an anionic exchange column, dialyzer, and charcoal column to detoxify the solution.27 Single-Pass albumin dialysis (SPAD) is similar to the above but is more simple because of the use of readily available hemodialysis equipment with the addition of albumin to the dialysis solution. Prometheus uses a system where a biocompatible filter is used to detoxify endogenous albumin, which is then purified by using a resin adsorber and anion exchanger33. The last step of detoxification uses high-flux hemodialysis.27,28
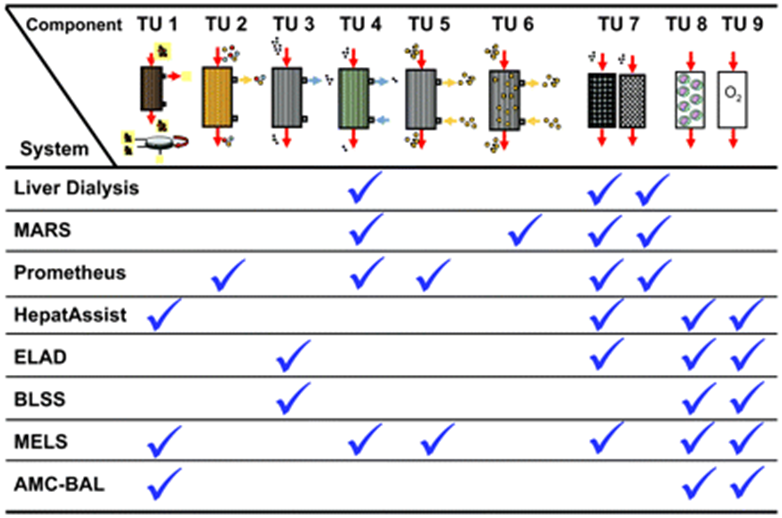
Figure 10 Summary of the treatment units integrated within each artificial or bioartificial liver support device.16
While these artificial devices have shown positive results, there has not been any indication of actual improvement of synthetic liver function.9,27,28 Detoxification may improve the condition of patients who are going through liver failure and may prolong the amount of time a patient can survive on their failing liver while waiting for a transplantation.9,28 MARS, Prometheus, and SPAD have shown success if reducing bilirubin and bile acids levels but demonstrated improvement in mortality rates has failed to be shown.9,27 Liver dialysis using hemodiabsorption was approved for use by the FDA in 1997 for encephalopathy treatment secondary to liver failure, but this treatment is not currently marketed because it is being redesigned.9,28 MARS is currently approved for use in the US by the FDA for the treatment of acute liver failure but is not defined as an alternative to liver transplantation.28 The conclusion is that more studies are needed to improve these devices and show functional results in improving cirrhosis and liver function (Table 2).
|
Device type |
Composition |
Advantages |
Disadvantages |
|
Artificial |
N / A |
- ease of use |
only detoxification |
|
Bio - Artificial |
Cells : |
- hepatic function |
- cell source not |
Bio-artificial devices
Bio-Artificial liver devices are hybrid systems that purify the blood and replace hepatic synthetic function (Figure 10).5,28. These devices use bioreactors that contain functional hepatocytes to provide active detoxification and biosynthetic hepatic functions.5,27 The most commonly used cell sources include autologous cells from the patient, allogenic cells from donors, allogenic cell lines, and xenogenic cells from various species (Table 3).9 Specific cells used include hepatoblastoma cell lines or porcine cells, but each comes with its disadvantages.28 Human cells could induce tumor growth and/or apoptosis, or lose detoxification and metabolic function over time.28 In addition, issues arise concerning distribution to the treatment site because of their magnitude.28 Porcine stem cells have greater potential because they are readily available and easily distributed, but risks include xeno-response and retroviral transmission.28 The scaffold that is used needs to preserve cell morphology and metabolism and allow for necessary mass transport to maintain cell health.9,28 Immune rejection needs to be avoided by the separation of cells from the blood by a porous material that allows for the passage of substances like toxins and synthesized proteins.9,28 The different BALs that are available include hollow fiber devices, packed beds, flat plate systems, and encapsulation-based reactors.5
|
Cells |
Source |
Advantages |
Disadvantages |
|
Autologous cells |
Patient |
- no immunotoxicity |
- limited availability |
|
Allogenic cells |
Donor |
- high availability |
- disease transmission |
|
Allogenic cell lines |
- |
- high availability |
- function loss |
|
Xenogenic cells |
Various Species |
- high availability |
- animal pathogen |
Hollow fiber devices are most common and use cartridges with hollow fiber membranes that allow for hepatocyte adesion. The fiber membranes are the scaffolds for cell attachment and compartmentalization. Examples of this system include the Extracorporeal Liver Assist Device (ELAD) and HepatAssist. ELAD uses the hollow fiber technique in combination with charcoal resins and oxygenation to separate functional cells from the patient’s plasma.5,28 Human hepatoblastoma cell lines have been used to detoxify and maintain oxygen supply to the functional cells.5,27 There have been clinical trials limited to ALF patients that have demonstrated safety but were not able to show survival advantage.5,27,28 The first device to advance to phase II and III clinical trials is HepatAssist.5,9 The system uses porcine hepatocytes contained in a hollow fiber bioreactor to regenerate hepatic function and resin column to detoxify patient plasma. There have been limited studies, which report device safety, but not survival improvement or advantage. Other products include the Bioartificial Liver Support System, Amsterdam Medical Center-Bioartificial Liver, and Modular Extracorporeal Liver–all of which have extremely limited case studies and clinical experience (Table 4). Overall, there is great potential for cirrhosis treatment with BAL systems, but challenges with cell sources, loss of cell viability and functionality, large-scale cell culture, regulatory limitations, and cost issues have greatly limited their use (Table 2).5,9
Pipeline products
Many different techniques including fabrication technologies, cell-based technologies, microfluidic systems, and extracorporeal liver devices are being applied in tissue engineering for the liver (Table 5). There are very few clinical trials that have shown successful tissue regenerative products for liver failure and cirrhosis. Many trials listed in the United States are testing drugs for the treatment of primary diseases or for post-liver transplantation. Biosynthetic liver scaffolds are being studied in order to create the ideal scaffold that mimics the native liver ECM. Scaffolds need to have a very high level of porosity to allow for blood-hepatocyte the nutrient exchange, as well as a large surface area to volume ratio. Synthetic or biological scaffolds can be used. Synthetic hydrogel tuning allows for various degradation properties which can have advantages over biological scaffolds. They are reproducible, induce less immune responses, and have stable mechanics, but they are not widely used for liver tissue engineering in clinical applications because they are less bioactive and lack viscoelasticity.5,29 A few different approaches will be discussed below.
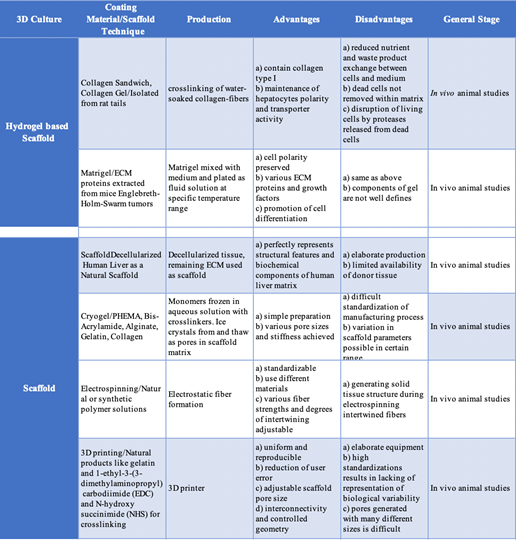
Table 5 Tissue Engineered Liver Products: Hydrogel-based versus non-Hydrogel scaffolds16
Tissue engineering liver products
The first is the decellularization/recellularization approach. The principle liver tissue engineering materials should be derived from humans and decellularized organs can be used as a scaffold because of the specific mocrosctructure. Decellularization is the process of removing cells and other immunogenic factors from the liver so the natural scaffold remains (Figure 11). This is the only scaffold that completely preserves the complex vasculature and biliary system, which is extremely important when trying to derive a liver-like structure.5,30 Decellularized human livers have been recellularized with hepatic stellate cells, hepatocellular carcinoma, and hepatoblastoma cells.5 The decellularization process should not damage the ECM and, after recellularization, should be able to functionally mature in a perfusion bioreactor. A study completed in 2010 (Uygun et al.), used a murine model to successfully complete the first recellularization of an acellularized liver transplanted. The process allowed for adequate function of the implanted hepatocytes on the 3D scaffold.
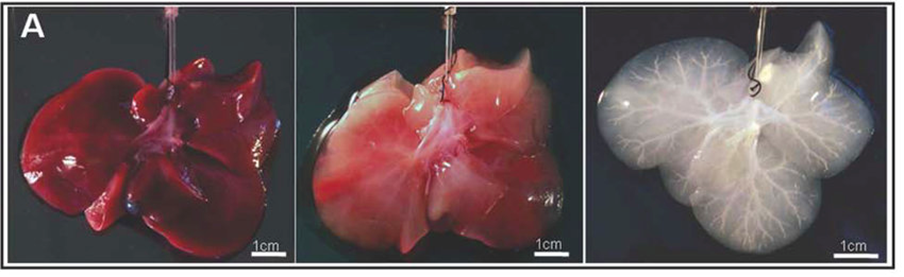
Figure 11 Liver tissue during various states of decellularization: native (A), partially decellularized (B), and fully decellularized (C).28
Cell encapsulation avoids the induction of an immune response and immunosuppression by immobilizing cells in a semi-porous scaffold and delivering biological products to patients.5,31 This approach meets all the necessary criteria for translational medicine: biocompatibility, acceptable microenvironment, cell survival, and cell proliferation and functionality. Recently, human-induced pluripotent stem cell-derived hepatocyte-like cells were cultured with human stellate cells and encapsulated in alginate beads.5 The study demonstrated improved and efficient iPSC differentiation compared to 2D monoculture conditions and structure implantation did not elicit an immune response in mice16. Acellularized ECM from the liver is another method for cell encapsulation because it can allow for the necessary interactions between the implanted cells and ECM. There need to be more studies for long-term in vitro maintenance and in vivo transplantation for clinical approaches.5 A few challenges arise with this method including induction of an immune response and toxicity of crosslinking components.
3D bio-printing shows promising applications for liver tissue engineering. This technique fabricates biomimetic self-assembling contracts using spheroids to create the organ. The liver is a more complex organ to print because of its complexity.32 The process suspends live cells in a bio-ink that can be cross-linked during or after the bio-printing process to shape the structure of desired organ. The bio-inks are made from various materials that can be natural, synthetic, or a combination of both.5 Various 3D-bio-printing technologies have been utilized including ink-jet-based, laser-assisted, extrusion-based, stereo-lithography, and microvalve-based. Bio-printing in combination with bioreactors allows for high-throughput fabrication of complex and controlled 3D structures which can be used for organ-on-a-chip platforms.5,33 Overall, this technique can improve the maturation of hepatocyte-like cells, as well as preserve ex vivo hepatocyte function and maintenance.
Microfluidic systems have a technology referred to as organ-on-a-chip that mimics a micro-environment to build functional units. In less than 7 years, over 28 companies have been registered for organ-on-a-chip.5,34 Liver-on-a-chip systems have been able to foresee toxicity and improve certain drug sensitivities. Up to 4 different types of primary murine hepatic cells have been integrated into an in vitro liver chip. This chip showed similar liver physiological cell composition, mechanical properties, and structure. While this technique is still being developed, it shows very optimistic predictions of drug toxicity.5
Over the past 5 years mortality due to cirrhosis has dramatically increased, pushing the healthcare industry to find new treatments for the disease.1 Orthotopic liver transplant is the only known effective treatment of cirrhosis and liver diseases, but this is limited because of organ donor shortages.5 The main goal currently is to detect end-stage liver disease as soon as possible in order to prevent the progression and subsequent complications caused by cirrhosis43. Many novel drugs and vaccines are being distributed across the world in order to reduce the burden of viral-related liver diseases in both underdeveloped and developed countries alike.20 With hundreds of thousands of people worldwide dying from lack of adequate treatment, a replacement for liver transplantation with reliable and accessible methods is needed. Promising solutions lie in liver tissue engineering and regenerative medicine as they have shown great efficacy in other healthcare markets.5
While dialysis-based technologies for liver disease treatment have been introduced in the public market, many have not demonstrated improvement in patients with cirrhosis. Novel techniques including in vitro modeling, artificial liver, cell encapsulation, organ decellularization, 3D printing, and organ on a chip have risen as contenders for this technology. While these methods are still in the preliminary stages and have many limitations that need to be addressed before clinical application, there is hope for demonstrated treatment in the future. Most importantly, the mechanical and physical properties of synthetic-based and biologic-based scaffolds must be improved to support nutrient exchange among cells, metabolic activity, differentiation, and survival while reducing immune response and toxicity. Finally, it is critical for scaffolds to exhibit similar structural properties to the liver ECM because of their complex vascular structure and tissue properties.5
Taken together, these findings indicate that a significant amount of work needs to be put in to develop tissue engineering methods for the liver. A small portion of the products and devices detailed in this paper has gone through proper clinical and regulatory testing, denoting the lack of efficacy and quality for their intended use.24 The main issue lies in large production costs and upscaling for the production of tissue-engineered products.35 Increased collaboration between regulatory agencies, industry stakeholders, and clinical bodies has allowed for expedited commercialization and approval pathways, which will hopefully mitigate the various TE application difficulties outlined in this review.35–43 While the market for liver tissue engineering needs time for growth, there are vast possibilities for various technologies to bridge liver transplantation for cirrhosis and end-stage liver disease.44–53
The author would like to thank Dr. Bill Tawil for his support in the course and for encouraging innovation and learning.
Authors declare that there is no conflict of interest.
There is no funding to report for this study.

©2023 Steward, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.