Journal of
eISSN: 2572-8466


Research Article Volume 9 Issue 5
1School of Physical Sciences, Jawaharlal Nehru University, India
2Department of Chemistry, School of Chemical and Life Science, India
3State Physics Division, Bhabha Atomic Research Centre, India
4Laboratory for Neutron Scattering, Paul Scherrer Institut, Switzerland
Correspondence: Himadri B Bohidar, School of Physical Sciences, Jawaharlal Nehru University, New Delhi, India
Received: September 08, 2022 | Published: September 19, 2022
Citation: Pandey PK, Rawat K, Aswal VK, et al. Hierarchical self-assembly in ionic liquid crosslinked polyelectrolyte gels as a citric acid sensor. J Appl Biotechnol Bioeng. 2022;9(5):142-147 DOI: 10.15406/jabb.2022.09.00302
The design of an enzyme-free biocompatible electrochemical platform for the detection of citric acid is discussed. We have studied sol-gel transition in DNA ionic liquid solution triggered by the ionic liquid (IL), (1-Octyl-3-methylimidazolium Chloride [C8mim][Cl]; concentration= 0.1 to 1.0% (w/v)). Rheology and Small-angle neutron scattering (SANS) studies characterized their viscoelastic and microstructural properties. The gel modulus was found to vary from 10 to 60 Pa concomitant with a tunable temperature of gelation. SANS data yielded the persistence length of DNA (50 ± 12 nm) and the mesh size of these gels (ξ = 2.5±0.5 nm). Remarkably, this ionogel served as an excellent electrochemical sensor for citric acid detection in the application window of 0.02-0.08 mM analyte concentration with a detection limit of 1.510-8 molL-1. Yet another non-trivial application of the versatile DNA gel is demonstrated here.
Keywords: ionic liquid, DNA, rheology, SANS, electrochemical sensor
DNA, deoxyribonucleic acid; EGDA, ethylene glycol diacrylate; IL, ionic liquid
Deoxyribonucleic acid, DNA being a highly functional molecule with excellent structural versatility, its derivatives like hydrogels, interpolymer complexes, condensates, etc. have continued to draw much attention which is evident from the following examples. The behavior of pH-triggered fast responding DNA hydrogel was demonstrated for sustainability under different pH conditions, which is required for the tailored application of this gel in biology, in particular.1 Different length of DNA chains have been shown to form a stable assembly with cationic colloidal particles.2 Graphene oxide was used to form multifunctional DNA hydrogel with high rigidity, biocompatibility, and self-healing property.3 Supramolecular polypeptide -DNA hydrogel with high rigidity modulus was used for 3D printing.4 DNA is polyanionic in nature and the double helix strands repel each other because of Coulombic repulsion. Therefore, to facilitate physical entanglement a cross-linker such as ethylene glycol diacrylate (EGDA) is often used.5 Polymerase enzyme-based DNA meta-hydrogel could be used in electrical circuits in which water played the role of an ON-OFF switch.6 Interaction of DNA and multi-walled carbon nanotube leads to the formation of self-assembled DNA-hybrid hydrogel.7 Comparative interaction study of ds- and ss-DNA with cation was extensively explored and it was concluded that ss-DNA collapsed more than the other.8 DNA functionalized hydrogel was successfully used to detect mercury from the environment.9 Stable DNA hydrogel fibers could be prepared in the presence of ionic liquid without requiring high temperature.10 Surfactant CTAB, and lysozyme protein enabled the assembly of DNA gel particles with ss- or ds-DNA.11 DNA hydrogel was shown to entrap gold nanoparticles inside its network.12 Strain hardening was noticed in both chemical and physical DNA gels at 40 % deformation.13 Cross-linking of DNA gel with organic salt such as CaCl2 showed reduced osmotic pressure.13 Relaxation dynamics and rheological properties of DNA hydrogels were systematically explored and microscopic changes were probed by neutron scattering technique.15,16
On the other hand, gelation in ionic liquid solutions is not uncommon. Sui et al have shown that poly(ferrocenylsilane) in the presence of polyionic liquid forms nanogels.17 It was seen that block copolymer forms self-assembled spherical or worm-like micelles in ionic liquid.18 Ionic liquid-based biopolymer gels typically have very high viscosity and melting temperature. Further, enabled by hydrophobic hydration, polymers like cellulose, which is generally insoluble in water and most other solvents, can be dissolved in ionic liquids.19 Even at very low concentration of DNA gelation was sustained in ionic liquid solutions.20 Self-assembly of the triblock copolymer was studied with the help of rheology and it was found that gels formed in the presence of ionic liquid were stable even at higher temperatures (100 0C).21 Pandey et al have demonstrated the formation of DNA ionogels and studied their time-dependent structural dynamics, viscoelastic properties and microscopic structures.22 These ionogels have found applications in the production of lithium-ion batteries, fuel cells, solar cells, electrochemical sensors, actuators and also as drug encapsulation and release vehicles.23 Herein, we have studied 1-octyl-3 methylimidazolium ionic liquid-based DNA ionogels and these have been used to propose an electrochemical biosensor for the important nutrient citric acid. This is a weak organic acid with molecular formula C6H8O7.
2000 base pair Deoxyribonucleic acid (DNA), sodium salt from Salmon testes (Catalog no. D1626) and the ionic liquid (IL) 1-octyl-3-methyl imidazolium chloride [C8mim][Cl] were purchased from Sigma-Aldrich (USA). Potassium ferrocyanide, K4Fe(CN)6 and Potassium ferricyanide, K3Fe(CN)6 were purchased from SRL (India). Potassium Chloride and KCL were from CDH (India) and so were the analytes Cholesterol (Chox), Citric acid (CA) and Gallic acid (GA). Urea (U) and Dextrose (Dex) were purchased from Loba Chemie (India). Oxalic acid (OA) was purchased from RANKEM (India). Indium-tin-oxide (ITO, 1.1 mm thick and 25 Ω sq-1 resistance) substrate was purchased from Balzers, UK (Baltracom 247 ITO). All measurements were performed at room temperature 250 C unless otherwise stated.
DNA was dissolved in different concentration (0.1% to 1.0 % (w/v)) of ionic liquid aqueous solutions in such a way that final concentrations of DNA remained fixed at 1% (w/v) in all the samples. These samples were heated to 90 0C and maintained at that temperature for 30 min. Then, on cooling to room temperature optically clear gel samples were obtained. Since, these gels were made in ionic liquid solutions, these are being referred to as ionogels.
Viscoelastic properties of ionogels were measured by AR 500 Rheometer, TA Instruments, (England) using a 20 mm and 20 cone-plate geometry. Small angle neutron scattering (SANS) measurement was carried out at the Swiss Spallation Neutron Source SINQ, PSI (Switzerland) facility using beam wavelength of 0.8 nm.24 Electrochemical sensing of different analytes was performed on a cyclic voltammeter (CV) on Auto lab Potentiostat/ Galvanostat (Eco Chemie, Netherlands).
The thin film of DNA ionogel-ITO electrode was prepared by the drop casting method, where a drop of the ionosol was uniformly coated by dispersion of 50 µl of the suspension onto an ITO plate that had a covered surface area of 0.25 cm2 which created a thin gel film. The films were then allowed to dry at room temperature for about 24 hours. The electrochemical behaviour of the prepared electrodes was monitored and the maximum current was detected for the sample with ionic liquid concentration of 0.7% (w/v). Except for a few experiments (standardization of IL concentration) all other experiments were performed on thin gel films containing 1% (w/v) DNA and 0.7% (w/v) IL.
Viscoelastic behavior
Rheological characterization of the samples were performed by following two distinct protocols:25,26 (i) isothermal frequency sweep studies where dispersion behavior of storage and loss moduluswere recorded (Figure 1a), and (ii) isochronal temperature sweep studies that yielded the melting profile of the gels (Figure 2a). The photograph of these samples is shown in Figure 1b which shows that the meniscus of the gel does not flow upon inversion of the test tube. In the linear viscoelastic regime the dependence of elastic modulus on frequency w is often given by the power-law relation 27,28

Figure 1 (a) Frequency-dependent behaviour of storage modulus G^' with different IL concentration and (b) photograph of sol and ionogels.
(1)
Least squares fitting of the data to above equation yields the exponent n = 0.8 ± 0.4 (Figure S1 (Electronic Supporting Information)). The characteristic exponent n lies between 0 and 2 and uniquely defines the viscoelasticity of the material. When n = 0, it refers to a Hookean solid whereas n = 2 corresponds to a Maxwellian viscoelastic material. The gel strength G0 can be determined from
(2)
The value of G0 changed from 10 ± 4 to 60 ± 10 Pa with increase in the IL concentration from 0.1% to 1.0% (w/v). Therefore, as IL concentration was raised there was a concomitant increase in the gel rigidity indicating the formation of a denser network (Figure 1).
A characteristic length scale called the viscoelastic length ξel can be deduced from the knowledge of G0. The elastic energy stored in the network of volume ξel3 is equal to the thermal energy for an equilibrium gel. Hence, ξel is given by29
(3)
For the 0.1% IL sample = 120 nm which reduced to 40 nm for 1.0 % IL sample (Figure S2 (Electronic Supporting Information) implying formation of high network density gels in sols that had a propensity of ionic liquids. Higher network density would mean a stronger gel which is depicted in Figure 2b.
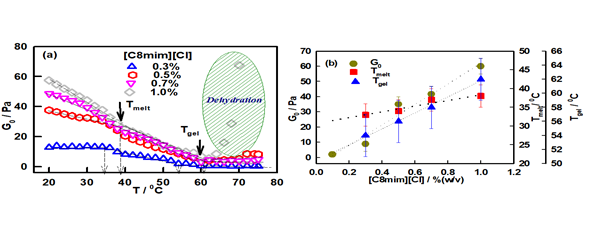
Figure 2 (a) Temperature-dependent profile for storage modulus G0. Change in slope depicts the transition temperature, referred as Tmelt and Tgel. (b) IL concentration-dependent variation of G0, Tmelt and Tgel.
The isochronal temperature sweep data of these gels clearly show two transition temperatures which are marked in Figure 2(a). The first transition point was found between temperatures 35-40 0C (Tmelt) whereas the second was located (Tgel) in the higher temperature region (53-62 0C). The dependence of Tmelt and Tgel on ionic liquid content is shown in Figure 2(b).
Note that the gel melting starts at Tmelt and ends at Tgel. From Figure 2 (b), it can be easily interpreted that all G0, Tmelt and Tgel are linearly dependent with IL concentration. The gelation is associated with enthalpy change conforming to the first order phase transition behaviour. Linear fitting of G0, Tmelt and Tgel data with respect to IL concentration, shows the change in these parameters per unit change in IL concentration (slope of the fitting line) is given by G0 = 67.16 Pa/ % (w/v), Tmelt = 7.66 0C/ % (w/v), and Tgel = 11.40 0C/ % (w/v). Hence, this ionogel can be a designated as a tunable gel where the gelation temperature can be tuned in the range of typically ~ 50 to 650 C which is remarkable for a biopolymer gel. This is a much sought after property in the design of encapsulation and release vehicles in drug delivery formulations.
Small angle neutron scattering (SANS)
The microscopic structure of ionogel was probed by small angle neutron scattering measurement. The I(q) vs q structure factor data is shown in Figure 3. The data was defined in two distinct regions decided by and
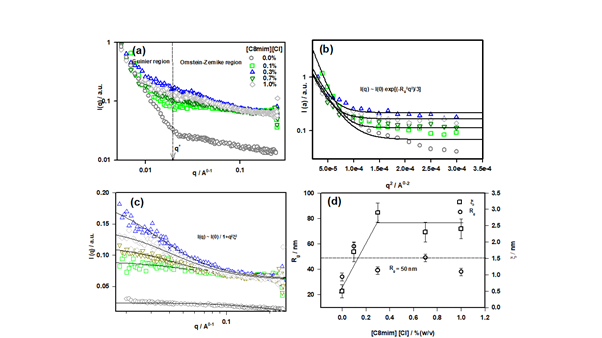
Figure 3 (a) I(q) vs q structure factor profile of different ionogels, (b) structure factor profile for low-q region fitted to Guinier function, (c) intermediate-q region fitted to OZ function and, (d) variation of parameters Rg and ξ with ionic liquid concentration.
protocols. These two regions were best fitted by Guinier law and Ornstein-Zernike (OZ) law.22 Guinier law was applied to the low-q region (qRg < 1), where Rg stands for the radius of gyration for the biopolymer. For the intermediate-q region, OZ function was applied for data fitting. The OZ equation describes the scattering from the dynamic network associated with the inter-chain overlap (i.e. the dynamic component). Therefore, yields the distance between two adjutant overlaps which is also called mess size (ξ) for the associated network. The low-q region (0.0552 Å-1 ≤ q ≤ 0.1173) was best fitted to the Guinier description and the intermediate-q region (0.0180 ≤ q ≤ 0.2480) to the OZ function. Therefore,
(4)
The first two terms of the above equation correspond to Guinier and Ornstein-Zernike function, respectively. In eqn. (4) IG and IOZ are q-independent pre-factors. Rg denotes the radius of gyration for the polymer and ξ is corresponding to correlation length or mess size of the gel network. The boundary between two regions is defined by a cross-over wave vector q* value (Figure 3a).
Form the data fitting, we obtained the value of Rg is 50 ± 12 nm which corresponds to the persistence length of DNA (~50 nm)30,31 and value of the mesh size was ξ = 2.5 ± 0.5 nm. The variation of these with ionic liquid concentration is shown in Figure 3d. The crossover wave vector q* was 0.019 leading to a cross-over length (L*= 2π/q*) of 33 nm sample (Figure S3 (Electronic Supporting Information).
Electrode characterization

Figure S3 Plots of relaxation time of fast modes with waiting time, tw shown for different IL concentrations.
The DNA Ionogel/ITO electrodes were characterized by Fourier transform infrared spectroscopy (FTIR) in the frequency range of 400 – 4000 cm-1 to investigate the changes that occurred after binding to the citric acid (Figure 4). We analyzed this on the basis in the variation in peak intensity. The DNA Ionogel/ITO electrode showed a broad peak located at 757.1 cm-1 arising from =C-H bending or/and C-Cl stretch. Peaks at 1217.6 cm-1 and 1361.9 cm-1 correspond to the imidazolium ring and the CH2 vibrational band. The peak at 1529.8 cm-1 arises because of C=C stretch, 1471.2 cm-1 is from C-H bending, and 1740.5 cm-1 mode is from C=O stretch is from the carboxylic acid functional group of citric acid. A peak at 3020 cm-1 originates from –N-H stretching, 3453 cm-1 is from -OH vibration and 3734.6 cm-1 is due to –NH stretching. Distinct changes in the spectra occurred on treatment with citric acid, where changes in the intensity of peaks were observed which established the interaction between the electrode and citric acid. A peak at 983.4 cm-1 is due to phosphate symmetric vibration within the ionogel. In the presence of citric acid, there was a major change or stretch in the peaks 1065 cm-1, 788 cm-1 and 1361.9 cm-1 (Figure 4).
The surface morphology of electrodes without and citric with acid was characterized using scanning electron microscope (SEM) imaging, which is shown in Figure 5a, which clearly reveals branched morphology of ionogel thin film. Image (b) shows surface morphology of electrode with citric acid. This change in surface morphology is caused by the transfer of citric acid to the ionogel interface on the electrode. Detail discussion of analytic detection is given later.
Optimization of parameters
For the electrochemical sensing of the analytes, a three-electrode cell was operated in the applied potential range of -0.05 to 0.5 V and in each case the redox current flowing in the cell was monitored. The three electrodes used were: DNA ionogel-ITO as working electrode, platinum wire as supplementary electrode and Ag/AgCl as reference electrode. All studies were conducted in standard Zobell's solution (3.3 mM K4Fe(CN)6, 3.3 mM K3Fe(CN)6 and 0.1M KCl) medium. Scan rate, ionogel concentration and analyte concentration were standardized by repeating the experiments thrice under similar conditions. Initially, we investigated the cyclic voltametric profile of bare ITO, and DNA ionogel-ITO electrode at a fixed scan rate of 50 mV/s in order to understand the electrochemical behavior in Zobell's solution and observed that the peak current of DNA ionogel/ ITO electrode was higher than the blank ITO electrode (Figure 6). This suggested the presence of a film on the ITO surface and there was a reduction in electron transfer accessibility by the gel film compared to ITO electrode.32,33
Later, the electrochemical response of DNA ionogel-ITO electrode was monitored systematically in the applied potential range of -0.1 to 0.5 V. As the concentration of IL in the ionogel electrode had a significant effect on the electrochemical properties, we first investigated this by varying IL concentration using peak current as an indicator. Figure 7(a) shows the CV profile of DNA ionogel-ITO electrode with varying IL concentrations. Figure 7(b) shows an increase in the anodic current Ia at IL concentration of 0.7% (w/v) and then it decreases, and remains invariant thereafter. As the highest peak current (Ia, anodic and Ic, cathodic) was obtained at 0.7% IL concentration indicating that this particular concentration had superior electron transfer properties and sensitivity compared to other samples. Therefore, all further experiments were performed using this particular electrode.
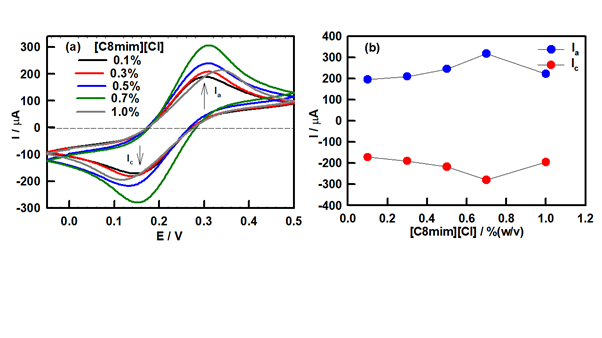
Figure 7 (a) Cyclic voltammetric response of ionogel electrode for different IL concentration varying from 0.1% to 1% (w/v), and (b) Electrode current (Ia and Ic) dependence on IL concentration in the ionogels.
The different analytes used in the study were urea (U), oxalic acid (OA), gallic acid (GA), cholesterol (CHOX), Dextrose (DEX), and citric acid (CA). Stock solutions of 10 mM of these analytes were separately prepared in deionized water and stored at 4 0C when not in use. The electron transfer kinetics of the electrode was analyzed using cyclic voltammetry. Initially, scan rate was optimized prior to performing studies on analytes. Thereafter, electrochemical studies were conducted for different analytes in the concentration range of 0.02-0.20 mM. The electrochemical sensing profile suggested that the DNA ionogel-ITO electrodes were only selective to citric acid (Figure 7).
Detection of analytes
The sole objective of the study was to analyse the potential utility of the DNA ionogel electrodes towards bioanalyte detection. We systematically probed the efficacy of these electrodes in the detection of various bioanalytes in the concentration range of 0.02-0.2 mM at a fixed scan rate of 50 mV/s by monitoring the change in the anodic and cathodic peak current. A single DNA ionogel-ITO electrode was used in the whole concentration range of a given analyte to the minimize instrumental error. Distinct changes in CV profiles were observed in the case of citric acid while no significant changes were seen in the case of other analytes. The anodic peak current was observed to increase with concentration of citric acid. Additionally, a shift in the anodic peak current towards higher potential was observed with increase in citric acid concentration. Figure 8a shows the CV response of DNA ionogel-ITO electrode to varying citric acid concentration. It clearly indicates an increase in the oxidation (Ia) and reduction (Ic) current on treatment with citric acid, and thus, infers that the electrocatalytic reaction yielding the redox current was due to the citric acid presence. No change in the peak current was found for all other bioanalytes even at very high concentration of 0.2 mM (data not shown).
On plotting the anodic and cathodic peak current for citric acid with respect to its concentration, a linear relationship was observed with a least-square fitting χ2 value of 0.97 and 0.98, respectively (Figure 8b). The linear detection range was found to be 0.02 to 0.08 mM and the sensitivity of electrode was calculated from the slope of current dependence of ionogel towards citric acid concentration (= 12.25 mA/mM cm2). Table 1 provides a comparative analysis of the different sensing platforms for citric acid detection and their relative sensitivity, which suggests that the present system offers a very high degree of sensitivity compared to the existing enzymatic and non-enzymatic modes of detection.33–37
S. No. |
Composition |
Linear range / M |
Detection limit / (mol L-1) |
References |
|
1. |
Cobalt(II)–phthalocyanine modified carbon paste electrode |
1 0.8–16.6 |
2.54×10-6 |
34 |
|
2. |
Ion chromatography with suppressed conductivity detection |
(10.4-5205)×10-7 |
3.13×10-7 |
35 |
|
3. |
Pyrolysis mass spectrometry |
5.21×(10-8-10-1) |
5.21×10-9 |
36 |
|
4. |
Reagent-injection spectrophotometry |
(3.0 – 600)×10-8 |
3×10-8 |
37 |
|
5. DNA based |
(0.02-0.08)×10-3 |
2.4×10-4 |
[Present Work] |
||
|
Ionogel/ITO |
|
|
|
|
|
|
|
|
|
|
Table 1 Comparison of different citric acid sensors
Enzymatic mode detection involving citrate lyase enzyme depends on the catalytic hydrolysis of citric acid. In the present method, a non-enzymatic approach is proposed where changes in electrochemical current were observed when the electrode was treated with increasing concentration of citric acid. Thus, a similar process to that of enzymatic reaction occurs where on interaction with the system, decomposition of citric acid produces oxaloacetate, which subsequently gets converted to COO-, that is responsible for the increase in peak current. A representative mechanism of citric acid detection by DNA ionogel-ITO electrode is presented in Scheme 1.

Scheme 1 (A) Gelation of DNA in ionic liquid solution, (B) DNA ionogel/ITO electrode (I) its preparation, (II) electrode in citric acid solution, (III) electron transfer of electrode surface and (IV) typical electrochemical response.
The interference data for citric acid sensor shows that the presence of another analyte do not affect the anode current Ia the sensor. Thus, it can be concluded that the sensor is specific to citric acid in the presence of other analytes of same concentration (Figure 9).
It has been shown that IL used can cause gelation in DNA solutions. It was noticed from rheology that these ionogels had moderate (10 to 60 Pa) rigidity modulus, but were endowed with tunable gelation temperature (52 to 62 0C). DNA being a strong polyelectrolyte binds to the positive head group of IL which forms DNA-IL complexes which in turn undergo gelation, because the electrostatic barrier of repulsion between inter DNA strands is depleted. The present study not only focused on the development of DNA ionogel system, but it was also used for the successful detection of citric acid. The electrode exhibited a very high degree of sensitivity of 12.25 mA mM−1 cm-2 in the linear range of 0.02-0.08 mM. This provides a new platform for the enzyme-free detection of citric acid, and holds promise for real-time use in clinical samples.
Supporting information (SI)
The variation of power-law exponent and viscoelastic length obtained from G0 obtained from frequency sweep rheology data for different samples are included in SI.
PKP acknowledge UGC-BSR, Government of India for Senior Research Fellowship. The authors thank the Advanced Instrument Research Facility of the University for TEM facility. KR thanks DST (SERB/EMR/2016/004868), ICMR (35/11/2019-Nano/BMS) and UGC-startup, Government of India for a research grant. This study was funded by DST-Purse II Department of Science and Technology, Government of India (DST), India.
The authors declare no competing interests.

©2022 Pandey, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.